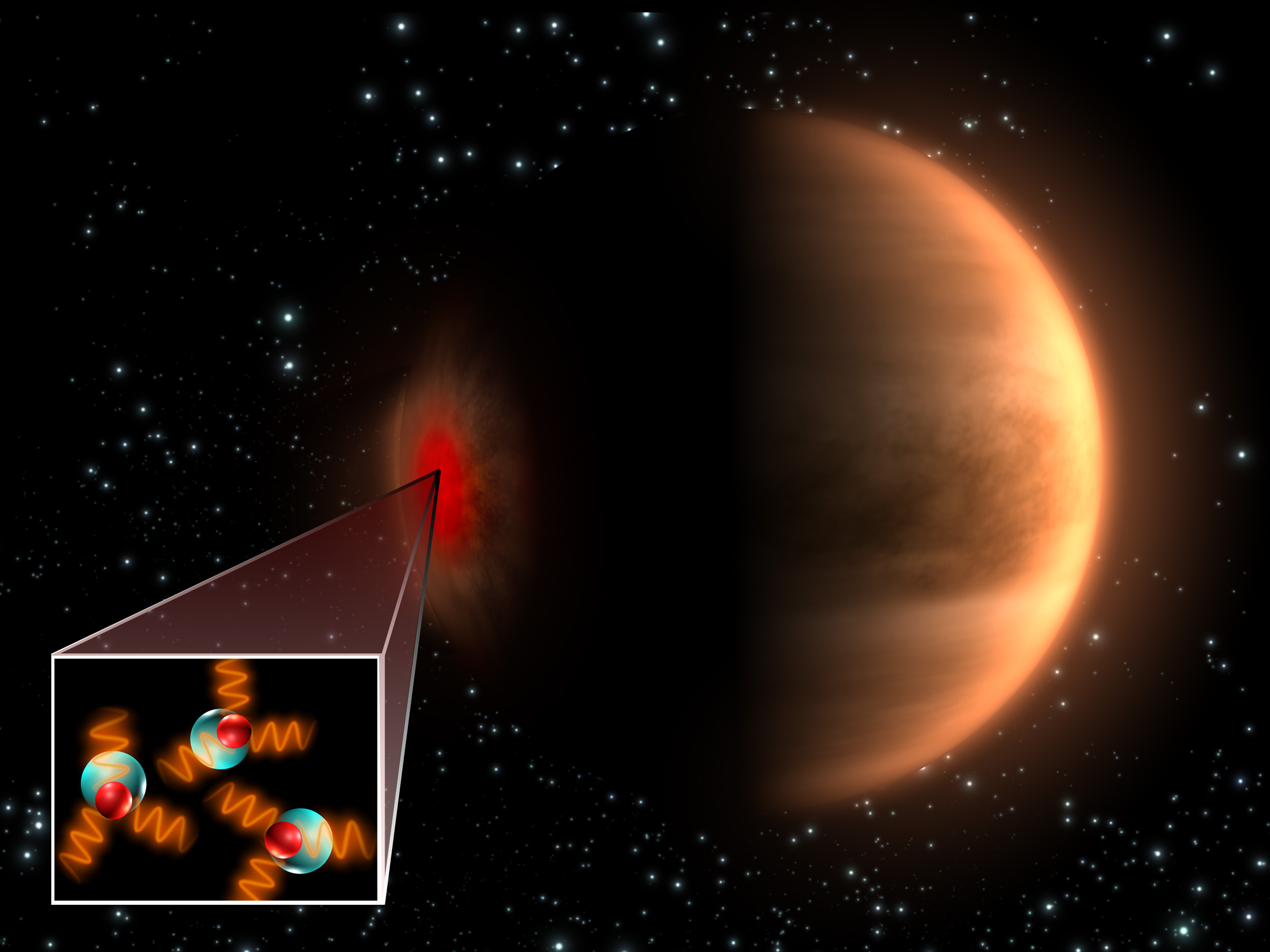
Hydroxyl, an important but difficult-to-detect molecule, has been found in the upper atmosphere of Venus by the Venus Express spacecraft. This is the first time this molecule has been detected on another planet, and even though it is thought to be an “atmospheric cleanser,” knowing that it is part of Venus’ thick, greenhouse-like atmosphere will help scientists better understand the environment on our next-door planet. “Venus Express has already shown us that Venus is much more Earth-like than once thought. The detection of hydroxyl brings it a step closer,” said one of the Principal Investigators of the VIRTIS experiment on the Venus Express, Giuseppe Piccioni.
Hydroxyl is thought to be important for any planet’s atmosphere because it is highly reactive. On Earth it has a key role in cleaning pollutants from the atmosphere. On Mars, scientists believe it helps stabilize the carbon dioxide in Mars’ atmosphere, preventing it from converting to carbon monoxide. Also, hydroxyl is thought to play a vital role in sterilizing the Martian soil, making the top layers hostile to microbial life.
Hydroxyl is made up of a hydrogen and oxygen atom each. It has been seen around comets, but the method of production there is thought to be completely different from the way it forms in planetary atmospheres.
On Earth, the glow of hydroxyl in the atmosphere has been shown to be closely linked to the abundance of ozone. From this study, the same is thought to be true at Venus.
Venus Express has shown that the amount of hydroxyl at Venus is highly variable. It can change by 50% from one orbit to the next and this may be caused by differing amounts of ozone in the atmosphere.
“Ozone is an important molecule for any atmosphere, because it is a strong absorber of ultraviolet radiation from the Sun,” says Piccioni. The amount of the radiation absorbed is a key parameter driving the heating and dynamics of a planet’s atmosphere. On Earth, it heats the stratosphere (layer of the atmosphere) making it stable and protecting the biosphere from harmful ultraviolet rays.
Computer models will now be able to tell how this jump and drop in ozone levels over short intervals affects the Venus’ restless atmosphere.
Original News Source: ESA Press Release

I’ve always wondered. How do they actually find elements on other planets and stars without physical experiment.
I’m not much of an expert. Pls forgive me for this silly question.
Cyber Dudezz,
The extremely short answer would be “spectroscopy.”
Cyber Dudezz Says:
May 15th, 2008 at 9:15 am
“I’ve always wondered. How do they actually find elements on other planets and stars without physical experiment.
I’m not much of an expert. Pls forgive me for this silly question.”
Chem Monkey Says:
May 15th, 2008 at 12:07 pm
Cyber Dudezz,
The extremely short answer would be “spectroscopy.”
Its not a silly question.
The slightly less-short answer is –
Basically we can detect and identify these chemicals through a process called spectroscopy – the study of the light emitted by the elements or molecules.
The basic principle is thus:
All elements have their own atomic structure, where structure here refers to the particular ways in which electrons are configured within the atom. An atom, in simple terms, is made up of a small positively charged nucleus and a cloud of electrons whizzing around it in ‘energy layers’. Electrons can often jump between these layers, and in the process either absorb or emit light – this is where light comes from.
The thing is, the wavelength of the light emitted (the colour of the light!) is completely determined by the energy levels that the electron jumps between to emit the light. In any given atom, there are a fairly large number of different energy levels that electrons can jump between, so the atoms emit light at a number of different and very specific wavelengths.
Whats more – each different type of atom (element) has its own unique energy level structure, and so the combination of different wavelengths of light it emits (it’s ‘spectrum’) is unique to that element – it is basically a ‘fingerprint’ for the atom in question.
Most light doesn’t appear this way to the eye (ie to be composed of different colours) because it all blends in together, but using a technique called spectroscopy, we can separate the light out and see what different colours or wavelengths are present. When we do this, we can see the chemical ‘fingerprints’ for different atoms present in the separated spectrum, and hence deduce that the light must have been emitted by a particular type of element or molecule.
My explanation is most probably lousy, by try looking ‘spectroscopy’ up on wikipedia for a better explanation.
“Hydroxyl is thought to be important for any planet’s atmosphere because it is highly reactive….Also, hydroxyl is thought to play a vital role in sterilizing the Martian soil, making the top layers hostile to microbial life.”
How is making Mars’ soil hostile to life “important” and “vital”?
The definition of “vital” is “absolutely necessary or important; essential” and “indispensable to the continuance of life”
Vital is from the Latin vita – “life”
I think improper working is being bandied about here.
that was improper wording… damn typos… how ironic! 🙂
nice photograph of venus. thanks to technology.