Anyone eager for a comet countdown? It’s just a few days now until the Rosetta spacecraft arrives near Comet 67P/Churyumov–Gerasimenko on August 6, and with each passing day more detail becomes visible.
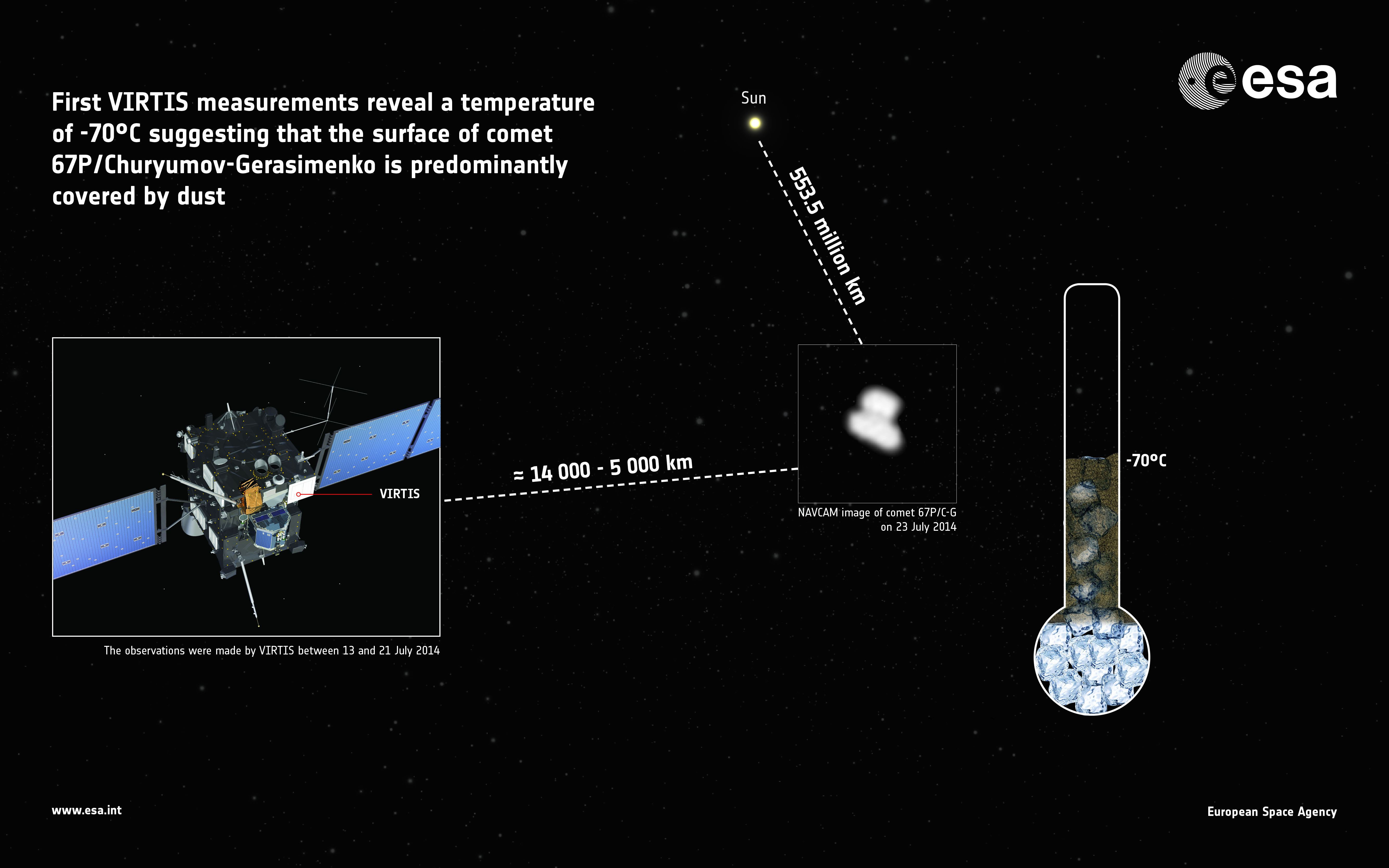
The “rubber duckie”-shaped comet has an average surface temperature of –70 degrees Celsius (-94 degrees Fahrenheit), which is far warmer than scientists expect. At 20 to 30 degrees Celsius (68 to 86 degrees Fahrenheit) warmer than predicted, the scientists say that the comet is too hot to be covered in ice. It must instead of a dark crust.
“This result is very interesting, since it gives us the first clues on the composition and physical properties of the comet’s surface,” stated Fabrizio Capaccioni, principal investigator of the visible, infrared and thermal imaging spectrometer (VIRTIS) that took the measurements.
Capaccioni, who is from Italy’s INAF-IAPS, led a team that took measurements of the comet between July 13 and July 21. What they found was also consistent with the findings from other close-up views of comets, such as 1P/Halley. Observations from afar already revealed that Rosetta had low reflectivity, so this is consistent with those far-off looks.
“This doesn’t exclude the presence of patches of relatively clean ice, however, and very soon, VIRTIS will be able to start generating maps showing the temperature of individual features,” stated Capaccioni.
Source: European Space Agency

I think some further explanation is in order, for us lay people. Why would a body with a surface temperature 70 degrees below the freezing point of water still be too hot to be covered in ice? I can speculate as to the reason for this counter-intuitive finding, but I really don’t know the answer for sure.
We’re used to thinking of the boiling point of water at 100 C, but keep in mind that this happens in one atmosphere of pressure. With lower pressures, the boiling point of water drops. That’s why, for example, liquid water is unstable at the surface of Mars despite a surface temperature below 0 C (the water would boil!).
In a vacuum, water has no liquid phase, and in the near-zero pressure, water would simply sublimate away at -70 C. If you do a Google Image search for “phase diagram of water” and look at the charts, this will illustrate it nicely.
Thank you, Thomas. I thought it had something to do with pressure and the vacuum but wasn’t sure.
Pete, another factor is that the ices within any given comet are not all water ice. Some of it is carbon dioxide ice or ‘dry ice’ which evaporates at much lower temperatures, -70.6*F and Carbon Monoxide which evaporates at -337.04*F. Those deposits are presumed to have mostly ‘boiled off’ the surface during earlier passages. The remaining dust and water ices above provides insulation for frozen CO2 & CO in pockets below the surface. Ablation of the surface dust and ices uncovers these deposits which may be responsible for some of the more explosive out-gassing events we sometimes witness.