
Outer space touches us in so many ways. Meteors from ancient asteroid collisions and dust spalled from comets slam into our atmosphere every day, most of it unseen. Cosmic rays ionize the atoms in our upper air, while the solar wind finds crafty ways to invade the planetary magnetosphere and set the sky afire with aurora. We can’t even walk outside on a sunny summer day without concern for the Sun’s ultraviolet light burning out skin.
So perhaps you wouldn’t be surprised that over the course of Earth’s history, our planet has also been affected by one of the most cataclysmic events the universe has to offer: the explosion of a supergiant star in a Type II supernova event. After the collapse of the star’s core, the outgoing shock wave blows the star to pieces, both releasing and creating a host of elements. One of those is iron-60. While most of the iron in the universe is iron-56, a stable atom made up of 26 protons and 30 neutrons, iron-60 has four additional neutrons that make it an unstable radioactive isotope.
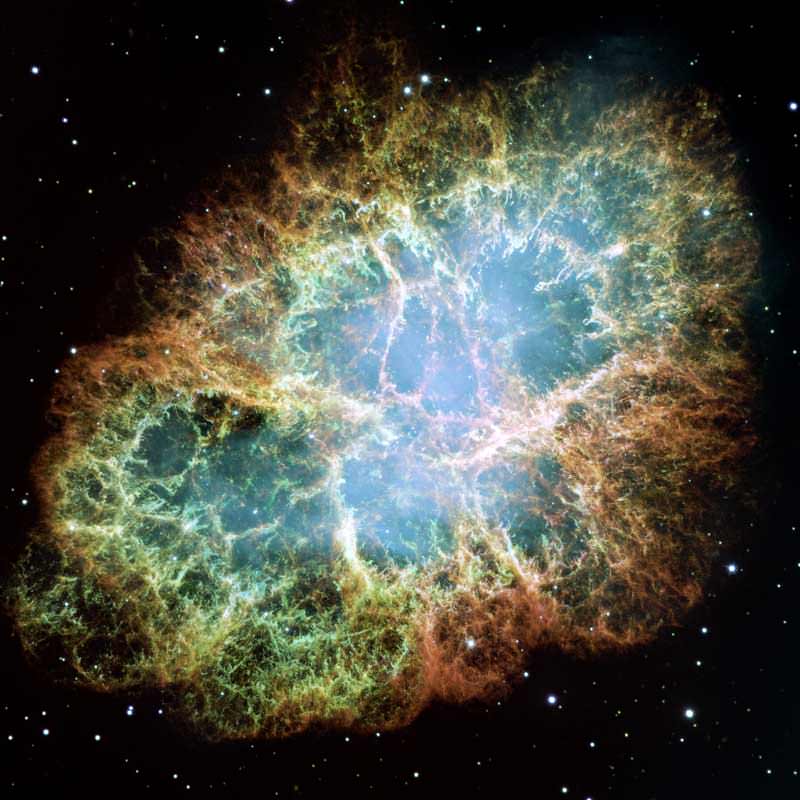
If a supernova occurs sufficiently close to our Solar System, it’s possible for some of the ejecta to make its way all the way to Earth. How might we detect these stellar shards? One way would be to look for traces of unique isotopes that could only have been produced by the explosion. A team of German scientists did just that. In a paper published earlier this month in the Proceedings of the National Academy of Sciences, they report the detection of iron-60 in biologically produced nanocrystals of magnetite in two sediment cores drilled from the Pacific Ocean.
Magnetite is an iron-rich mineral naturally attracted to a magnet just as a compass needle responds to Earth’s magnetic field. Magnetotactic bacteria, a group of bacteria that orient themselves along Earth’s magnetic field lines, contain specialized structures called magnetosomes, where they store tiny magnetic crystals – primarily as magnetite (or greigite, an iron sulfide) in long chains. It’s thought nature went to all this trouble to help the creatures find water with the optimal oxygen concentration for their survival and reproduction. Even after they’re dead, the bacteria continue to align like microscopic compass needles as they settle to the bottom of the ocean.
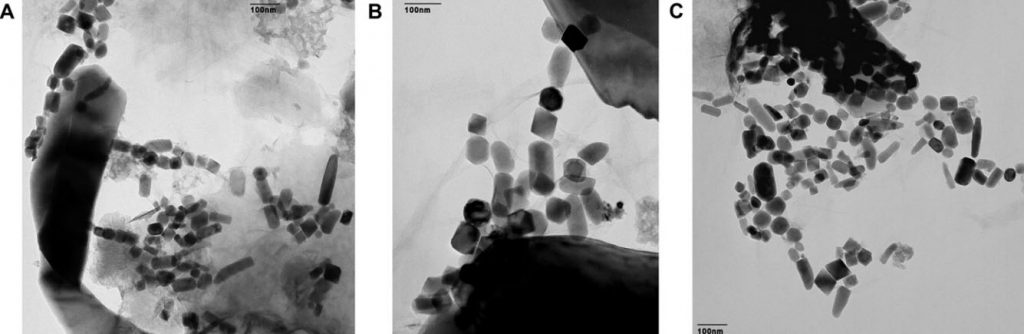
After the bacteria die, they decay and dissolve away, but the crystals are sturdy enough to be preserved as chains of magnetofossils that resemble beaded garlands on the family Christmas tree. Using a mass spectrometer, which teases one molecule from another with killer accuracy, the team detected “live” iron-60 atoms in the fossilized chains of magnetite crystals produced by the bacteria. Live meaning still fresh. Since the half-life of iron-60 is only 2.6 million years, any primordial iron-60 that seeded the Earth in its formation has long since disappeared. If you go digging around now and find iron-60, you’re likely looking at at a supernova as the smoking gun.
Co-authors Peter Ludwig and Shawn Bishop, along with the team, found that the supernova material arrived at Earth about 2.7 million years ago near the boundary of the Pleistocene and Pliocene epochs and rained down for all of 800,000 years before coming to an end around 1.7 million years ago. If ever a hard rain fell.

The peak concentration occurred about 2.2 million years ago, the same time our early human ancestors, Homo habilis, were chipping tools from stone. Did they witness the appearance of a spectacularly bright “new star” in the night sky? Assuming the supernova wasn’t obscured by cosmic dust, the sight must have brought our bipedal relations to their knees.
There’s even a possibility that an increase in cosmic rays from the event affected our atmosphere and climate and possibly led to a minor die-off at the time. Africa’s climate dried out and repeated cycles of glaciation became common as global temperatures continued their cooling trend from the Pliocene into the Pleistocene.
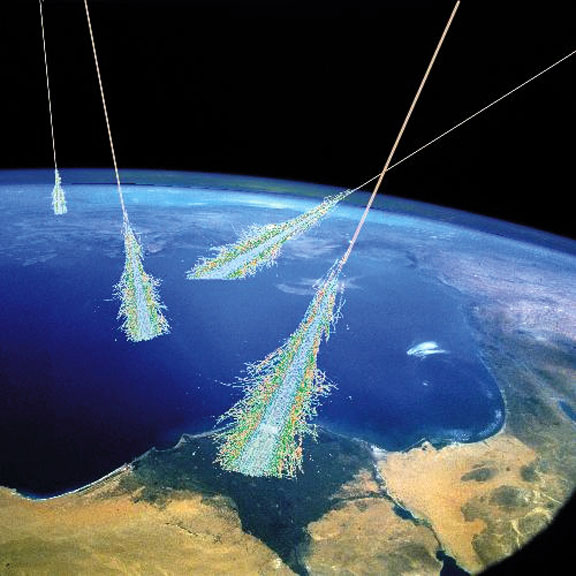
Cosmic rays, which are extremely fast-moving, high-energy protons and atomic nucleic, rip up molecules in the atmosphere and can even penetrate down to the surface during a nearby supernova explosion, within about 50 light years of the Sun. The high dose of radiation would put life at risk, while at the same time providing a surge in the number of mutations, one of the creative forces driving the diversity of life over the history of our planet. Life — always a story of taking the good with the bad.
The discovery of iron-60 further cements our connection to the universe at large. Indeed, bacteria munching on supernova ash adds a literal twist to the late Carl Sagan’s famous words: “The cosmos is within us. We are made of star-stuff.” Big or small, we owe our lives to the synthesis of elements within the bellies of stars.

Thinking about this article.. lots. Reminds just how precarious our existence actually is in this big old bad ass universe. Were a S/N to pop off nearby, from an unknown or unexpected source, it could make all of our/humanity’s efforts irrelevant? One wonders how many times this might have happened to other ‘advanced’ civilizations in our galaxy.. or elsewhere? Might be why we haven’t heard from anybody? ACK! Sounds kind of fatalistic, I know. But at least it would end our fears about Donald Trump getting elected! That worry pales in comparison? LOL! Arrrrgh…
In a lighter vIew, I would LOVE to see a S/N pop off in our galaxy… At a safe distance! I often complain about the light from a full moon blocking deep sky objects, but a S/N’s light doing the same for a couple months would be alright w/me!
Hi Bob,
Good article. One point to note – the light from the SN would have gotten here much before the Fe-60. The ejecta from the SN would be travelling at max 30,000 km/sec, while light is about ten times faster.
https://en.wikipedia.org/wiki/Supernova_remnant
So it’s reasonable to think that the SN was long gone before people encountered the Fe-60 rain.
Regards,
TB
This article is total BS our best global warming climatologists have ruled out earth being effected by anything outside of the atmosphere. . .