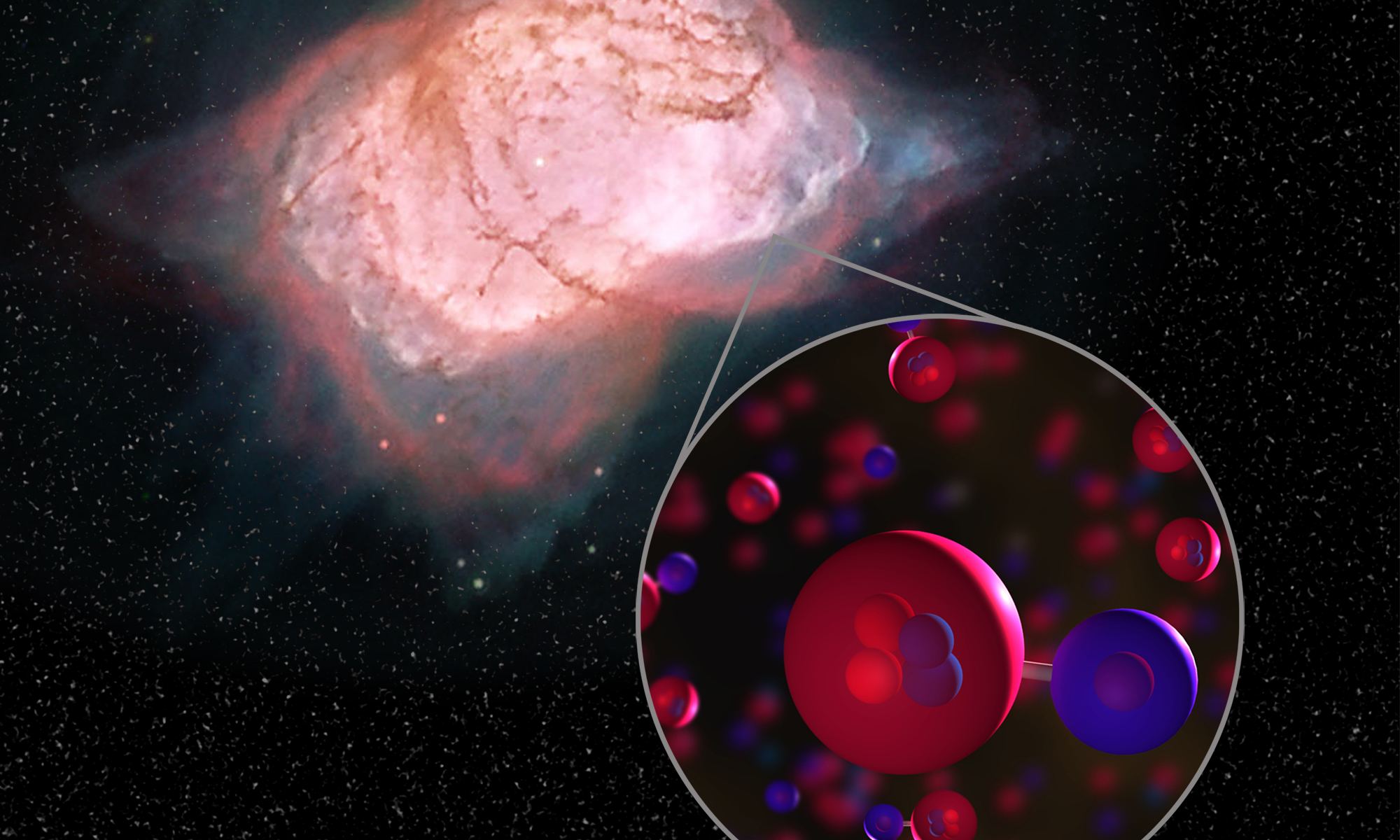
It takes a rich and diverse set of complex molecules for things like stars, galaxies, planets and lifeforms like us to exist. But before humans and all the complex molecules we’re made of could exist, there had to be that first primordial molecule that started a long chain of chemical events that led to everything you see around you today.
Though it’s been long theorized to exist, the lack of observational evidence for that molecule was problematic for scientists. Now they’ve found it and those scientists can rest easy. Their predictive theory wins!
In the very early days of the Universe, there were only two or three types of atoms. Hydrogen, helium, and tiny amounts of lithium were created by Big Bang Nucleosynthesis. All the other elements were forged later, in stars. Stars are mostly hydrogen, but stars couldn’t form from the simple hydrogen atoms created in the Big Bang. They form from what’s called molecular hydrogen. And molecular hydrogen couldn’t form without the so-called “first molecule,” a combination of helium and hydrogen called helium hydride. Theory says that helium hydride was created about 100,000 years after the Big Bang.
“It was so exciting to be there, seeing helium hydride for the first time in the data.”
Rolf Guesten, Max Planck Institute for Radio Astronomy, lead author.
You can picture a snap shot of the early Universe, somewhere around 100,000 years after the Big Bang. It was very hot, and was populated by only hydrogen, helium, and that tiny bit of lithium. Before the atomic population of the Universe could diversify, stars had to form. Once it began to cool, conditions were starting to get ripe for stars to form.
But something else had to happen, too. The cooling of the Universe wasn’t enough for stars to form. Molecular hydrogen had to be created, since stars are made largely from molecular hydrogen rather than the simple atomic hydrogen created by the Big Bang. (Scientists don’t call it simple hydrogen, they call it just a hydrogen atom.)
Most of the hydrogen in the Universe is molecular hydrogen.
But a single hydrogen atom is rare in today’s Universe, because it’s a free radical and is really reactive. Molecular hydrogen is a molecule in which two hydrogen atoms are bonded together. It consists of two protons and two electrons and is very stable. There are massive clouds of molecular hydrogen out there in space, and stars from those clouds.
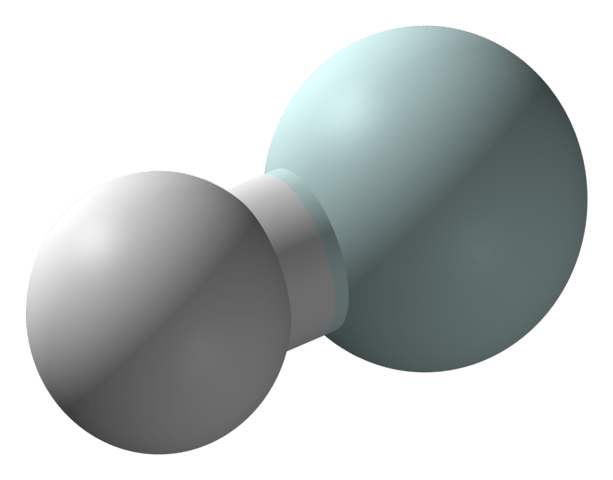
The problem in the early Universe was, even though things were cooling down, molecular hydrogen couldn’t form on its own. According to theory, simple hydrogen needed to interact with a specific molecule before it could form, and that molecule was helium hydride. This interaction was the first step in the chemistry of the Universe.
“The lack of evidence of the very existence of helium hydride in interstellar space was a dilemma for astronomy for decades.”
Rolf Guesten, Max Planck Institute for Radio Astronomy, lead author
Even though theory said that helium hydride had to exist, and even though it had been created in a lab in 1925, it had never been seen in space. It’s a very pickle molecule, because one of its constituent atoms is helium, a noble gas. And noble gases are very reluctant to react with other atoms.
But now they’ve found it.
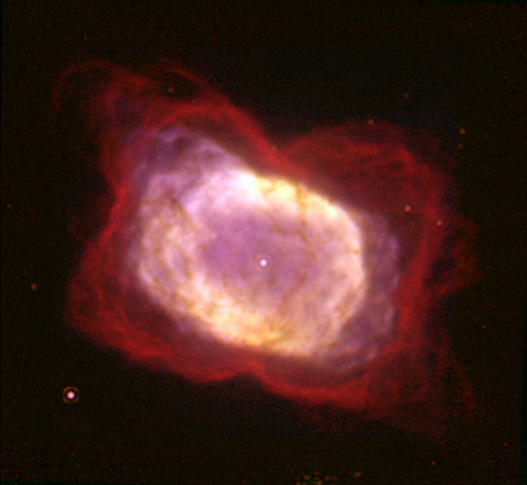
In a paper published on April 17th in the journal Nature, researchers outlined how they discovered the elusive helium hydride in the
planetary nebula called NGC 7027. They used NASA’s SOFIA, or
Stratospheric Observatory for Infrared Astronomy, to look for it. SOFIA is a converted Boeing 747SP which flies at high altitudes, above atmospheric interference, to make observations.
Ever since the 1970s, scientists thought that NGC 7027 had the necessary conditions for helium hydride to exist. Using SOFIA, and the German GREAT instrument (German Receiver at Terahertz Frequencies) they probed NGC 7027, searching for the elusive molecule.
Lead author of the paper is Rolf Guesten of the Max Planck Institute for Radio Astronomy, in Bonn, Germany. “The lack of evidence of the very existence of helium hydride in interstellar space was a dilemma for astronomy for decades,” said Guesten.
The planetary nebula where researchers spotted it has the right conditions for helium hydride to form. The aging star put out the right heat and ultraviolet radiation for the molecule to form. But looking inside that nebula proved to be very difficult. Enter SOFIA and GREAT.

SOFIA is kind of like a hybrid between a ground-based telescope and a space telescope. From its vantage point at 45,000 feet, it is free from most of Earth’s atmospheric interference, much like a space telescope. But it’s more flexible. It lands between missions and its instrumentation can be changed or adapted more like a ground-based telescope can.
In this case, the German GREAT instrument was integrated into SOFIA in 2011. And it’s proven to be pivotal in this research.
“We’re able to change instruments and install the latest technology,” said Naseem Rangwala, SOFIA deputy project scientist. “This flexibility allows us to improve observations and respond to the most pressing questions that scientists want answered.”
In 2016, scientists started using SOFIA and GREAT to probe NGC 7027 for the elusive helium hydride. Each molecule interacts with light on its own frequency, and GREAT was tuned to helium hydride’s frequency, similar to tuning a radio to a particular station. And they finally got lucky.
“It was so exciting to be there, seeing helium hydride for the first time in the data. This brings a long search to a happy ending and eliminates doubts about our understanding of the underlying chemistry of the early universe.
Rolf Guesten, Max Planck Institute for Radio Astronomy, lead author
“It was so exciting to be there, seeing helium hydride for the first time in the data,” said Guesten. “This brings a long search to a happy ending and eliminates doubts about our understanding of the underlying chemistry of the early universe.”
So this is the happy ending to one of astronomy’s longest-asked questions. The successful conclusion to the search for helium hydride is a nice victory for our theories detailing the evolution of the Universe.
If you’re friends with a scientist, go buy her a beer and show some appreciation for their hard work!
Sources
- Research Paper: Astrophysical detection of the helium hydride ion HeH
- Press Release: The Universe’s First Type of Molecule Is Found at Last
- NASA: SOFIA Website
- Wikipedia: Helium hydride ion