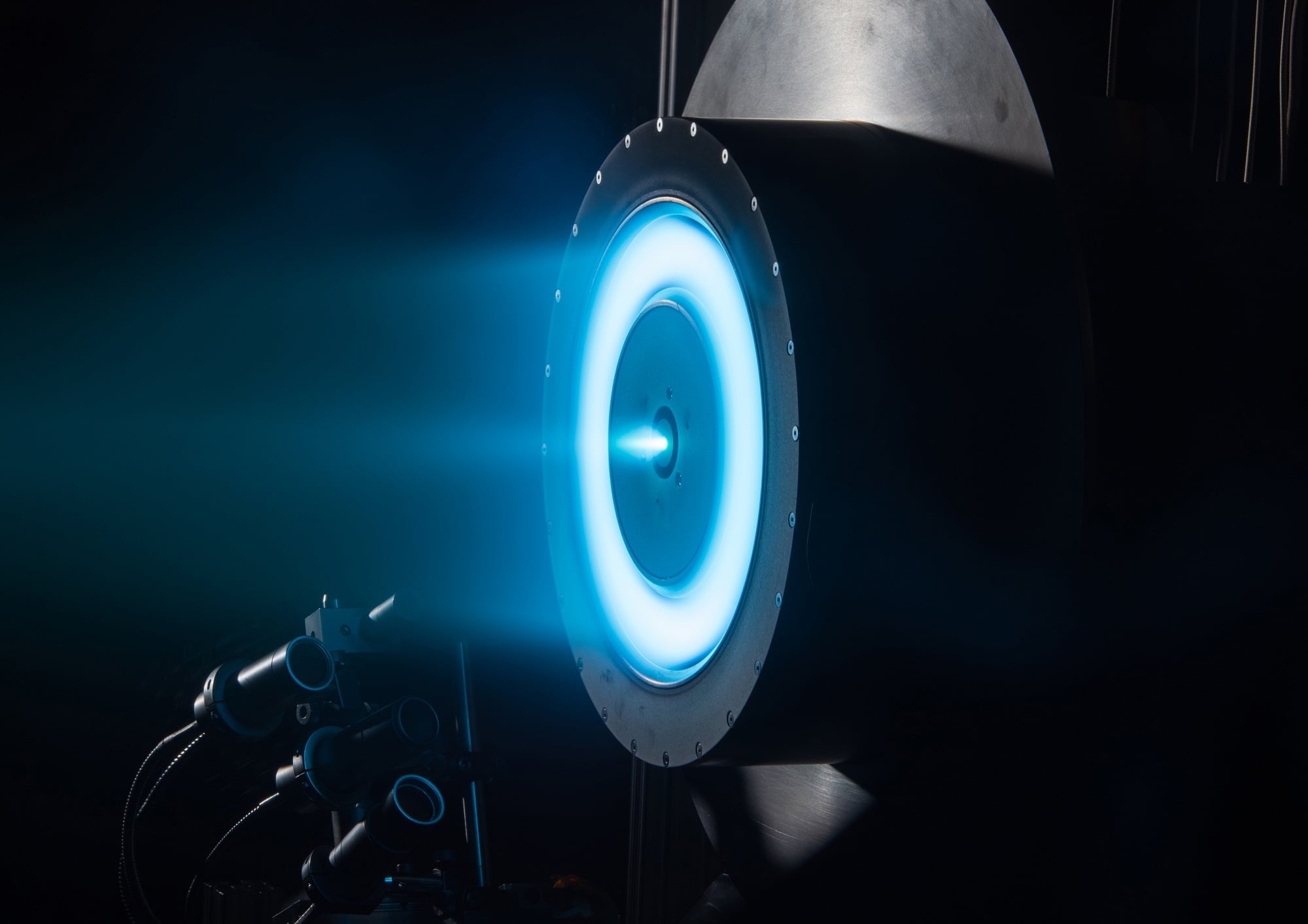
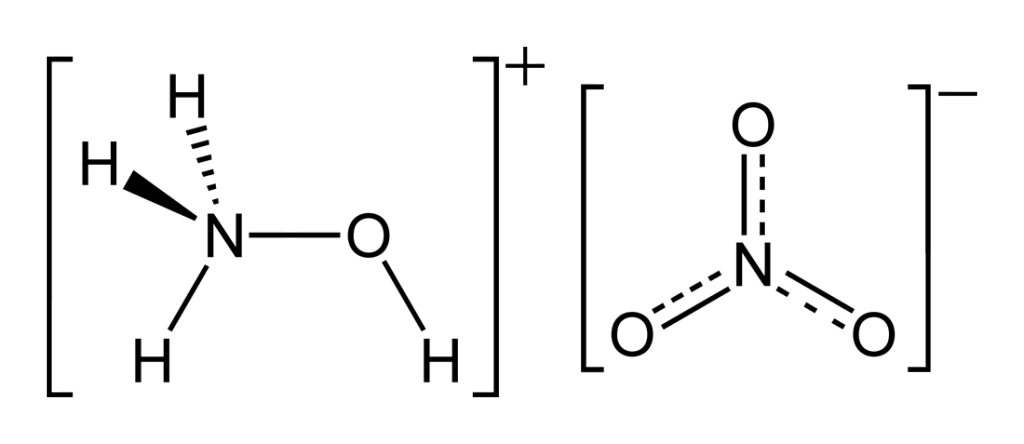Believe it or not there are some people out there who think traditional rocket science is too easy and want more of a challenge. A group at the University of Illinois (UI) decided to up the difficulty a bit by attempting to design a rocket engine that is capable of both electric and chemical propulsion.
Such a dual-mode rocket engine would have the benefits from both kinds of propulsion. The chemical side would give them significant thrust and quick reaction times when needed at the cost of efficiency, while the electric engine would allow for efficient, though slow, travel. Recently the group tested a novel type of rocket fuel that might just be able to be used in both types of engines.
A fuel that can be used in both electric and chemical propulsion has been a major sticking point in such "dual-mode" propulsion systems. Each type of engine requires very different chemical and electrical properties of it's fuel. For chemical combustion, a fuel must be able to ignite efficiently and at a reasonable speed. If it burns too fast and it will explode under pressure. If it burns too slowly the engine will just be shooting liquid fuel out it's nozzle.
Alternatively, for electric propulsion, most fuels are completely inert because the engine designers do not want them to accidentally ignite. Their electromagnetic properties are much more important. In order to be useful in an electrical propulsion system, a fuel must be capable of deforming in the presence of a strong electric field. As part of that deformation, some particles of the fuel will be pulled from the rest and will be ejected out of the thruster as ions.
The UI team might just have hit on a fuel that is capable of being used in both engines. It consists of a mixture of hydroxylammonium nitrate (HAN) and 1-ethyl-3-methylmidazolium ethyl sulfate (EMIM). These are both commercially available liquid ionic salts, but when combining them, Dr Joshua Rovey and his team might have hit on a solution to their long-standing dual-mode operational fuel.
One of the advantages of the salt combination is that HAN acts as a very good oxidizer, and is a known quantity in propulsion circles. EMIM on the other hand is a decent fuel. Since fuel and oxidizers are the two components needed to combust effectively, the combination of the two into a single fuel source allows for combustion to take place.
Since HAN was already a known quantity, the Rovey lab started to look for other salts that would mesh well with it. Their search was significantly narrowed by both commercial availability and availability of physio-chemical properties of the salt in the literature. EMIM was one of the three candidates they narrowed it down to, and the one that performed the best in preliminary combustion simulations.
One significant advantage the HAN/EMIM combination has over existing rocket fuels, such a hydrazine, is that it is non-toxic. But Dr. Rovey is quick to point out, "[Non-toxic] doesn't mean it's safe to drink - it means it's safe to breathe around." The combination is also denser than most accepted rocket fuels, meaning more fuel can be kept in a smaller volume - a critical concern when thinking about volume in a rocket's fuel tank.
That higher density doesn't necessarily mean it burns faster though. The paper the team just released focuses on studying what is called the "linear burn rate". This is a description of how fast the burning front of a liquid propagates into the rest of that liquid. As mentioned above, if that burn rate is too fast it could explode the entirety of the fuel tank. If it is too slow it will result in liquid fuel being thrown out of the rocket engine nozzle and of no use to anyone.
Burn rate is also affected by pressure, which is a concern when thinking about rocket engines, as the fuel must be pressurized to ensure it is effectively sent to the combustion chamber. It turns out the combination of EMIM and HAN have a burn rate right in the middle of the "Goldilocks zone" - not too fast and not too slow while under a reasonable pressure for potential use as a fuel source.
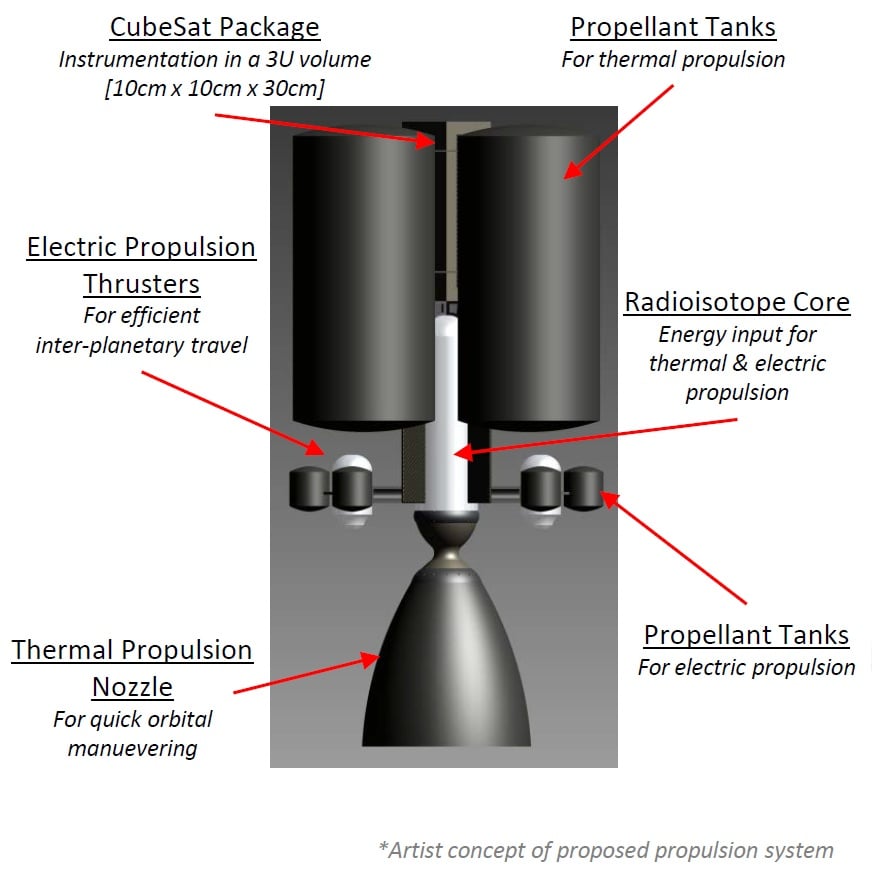
There's still a lot of work to be done though. Dr. Rovey's team has preliminarily proven that the salt mixture also works in electric propulsion. In addition, right now the team is at a phase where they are exploring a dual-mode concept with two separate thrusters for the two different propulsion methodologies, and they are also working on an even more challenging concept, a single thruster that is switchable between electric and chemical propulsion. Such a system would be most useful for systems as small as cubesats.
Dr. Rovey's team is actively working on testing and scaling up their research. They are actively funded by NASA and, while they don't have any technology demonstration flight planned for the propulsion system yet, they hope to have one scheduled in the next few years.
Those years will be filled with much more troubleshooting and engineering for the group. While there are still many hurdles to get to that point, developing and testing this novel type of non-toxic propellant is undeniably one step in that direction. Whether it will eventually lead to even more complicated rocket science remains to be seen.
Learn More:
EurekaAlert / University of Illinois: Under Pressure, nontoxic salt-based propellant performs well
Combustion and Flame: Linear burn rate of green ionic liquid multimode monopropellant
NASA: Dual-mode propulsion System Enabling CubeSat Exploration of the Solar System
 Universe Today
Universe Today