In-situ resource utilization is a hot topic these days in space exploration circles, and scientists and engineers have had a great advantage of getting access to new materials from bodies on the solar system that either have never been seen before, such as asteroids or haven't been visited in decades, such as the moon. Recently, China's Chang'e 5 brought back the first sample of lunar regolith to Earth in almost 50 years. Using part of that sample, researchers from several Chinese universities have developed an automated system to create rocket fuel and oxygen out of CO2, using the lunar regolith as a catalyst.
This process is particularly interesting because CO2 is known to exist on the moon. Back in 2009, LCROSS sensed some after impacting the moon's surface and analyzing the resulting plume of material. Rocket fuel and oxygen will be necessary parts of any significant space exploration effort, at least for the foreseeable future. So utilizing a ubiquitous material found on the lunar surface (regolith) to catalyze a CO2 reaction into these two valuable materials sounds like a win-win.
Specifically, the reaction using the regolith at a catalyst splits the CO2 into O2 and CH4, also known as methane, a potent greenhouse gas that also serves as an effective rocket fuel. Oxygen is no slouch in its own right when acting as rocket fuel, but it has other uses, such as being necessary for humans to breathe.
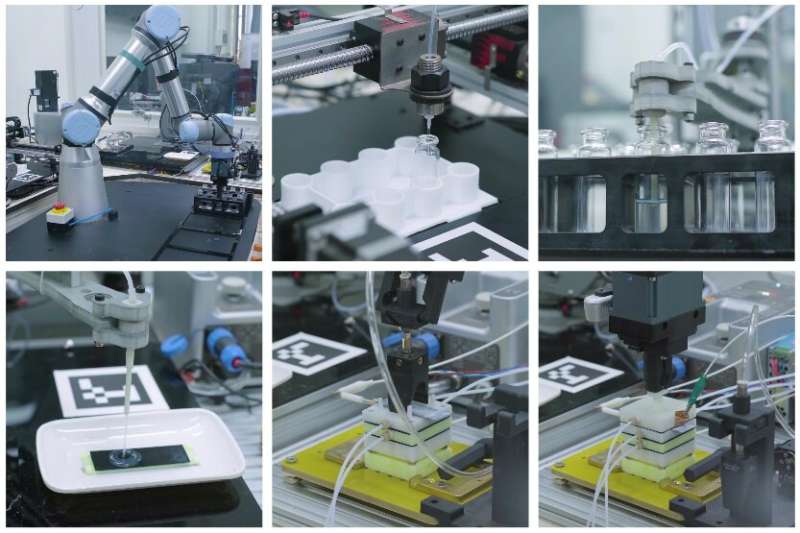
But the researchers didn't just stop at proving that lunar regolith can serve as a catalyst - they went so far as to automate the system completely. They reason that any base on the moon will have a severe workforce shortage for the foreseeable future, so any production reaction had to be completely automated.
And automate it they did, using a series of robots to transfer the necessary materials and provide the required power for the electrocatalytic reaction to take place. It seemed to work, as the system didn't require any human intervention to produce a relatively small amount of methane and O2 from part of the sample that Chang'e 5 brought back.
Whether or not this system would be advantageous over a more traditional hydrolysis process typically thought of as a source of both rocket fuel and breathable oxygen on the moon wasn't explored as part of this study. However, water, the basis for the hydrolysis process, has other uses as well, such as also being necessary to keep humans alive. On the other hand, carbon dioxide doesn't have as much use for humans, though plants are another matter if they ever exist in a large-scale moon colony.
Lunar regolith is hard to deal with in some situations. UT interviewed an expert to find out why.If any such colony ever exists, the current system will need a significant scaling up and plenty more raw feedstock to be a useful part of that colony. But any proof of concept like this is a step in the right direction leading towards that colonization. Now on to collecting some more moon dust to see what other experiments can be done with it.
Learn More:
Science China Press - Scientists investigate using lunar soils to sustainably supply oxygen and fuels on the moon
Yuan Zhong et al - In situ resource utilization of lunar soil for highly efficient extraterrestrial fuel and oxygen supply
UT - There’s Enough Oxygen in the Lunar Regolith to Support Billions of People on the Moon
UT - What is Lunar Regolith?
Lead Image:
Photograph (left) and scanning electron microscope image (right) of the lunar regolith used in the experiment.
Credit - Science China Press
 Universe Today
Universe Today