The origin of Earth's water has been an enduring mystery. There are different hypotheses and theories explaining how the water got here, and lots of evidence supporting them.
But water is ubiquitous in protoplanetary disks, and water's origin may not be so mysterious after all.
A research article in GeoScienceWorld Elements shows that other young solar systems have abundant water. In solar systems like ours, water is along for the ride as the young star grows and planets form. The evidence is in Earth's heavy water content, and it shows that our planet's water is 4.5 billion years old.
The article is " We Drink Good 4.5-Billion-Year-Old Water, " and the authors are Cecilia Ceccarelli and Fujun Du. Ceccarelli is an Italian astronomer at the Institute for Planetary Sciences and Astrophysics in Grenoble, France. Du is an astronomer at the Purple Mountain Observatory in Nanjing, China.
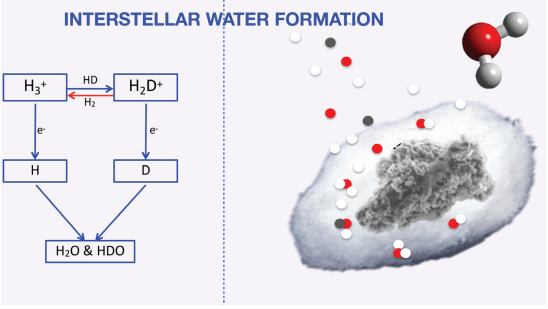
The formation of a solar system starts with a giant molecular cloud. The cloud is mostly hydrogen, water's main component. Next are helium, oxygen, and carbon, in order of abundance. The cloud also contains tiny grains of silicate dust and carbonaceous dust. The research article takes us through the history of water in our Solar System, and this is where it starts.
Out here in the cold reaches of a molecular cloud, when oxygen encounters a dust grain, it freezes and adheres to the surface. But water isn't water until hydrogen and oxygen combine, and the lighter hydrogen molecules in the cloud hop around on the frozen dust grains until they encounter oxygen. When that happens, they react and form water ice—two types of water: regular water and heavy water containing deuterium.
Deuterium is an isotope of hydrogen called heavy hydrogen (HDO.) It has a proton and one neutron in its nucleus. That separates it from "regular" hydrogen, called protium. Protium has a proton but no neutron. Both these hydrogen isotopes are stable and persist to this day, and both can combine with oxygen to form water.
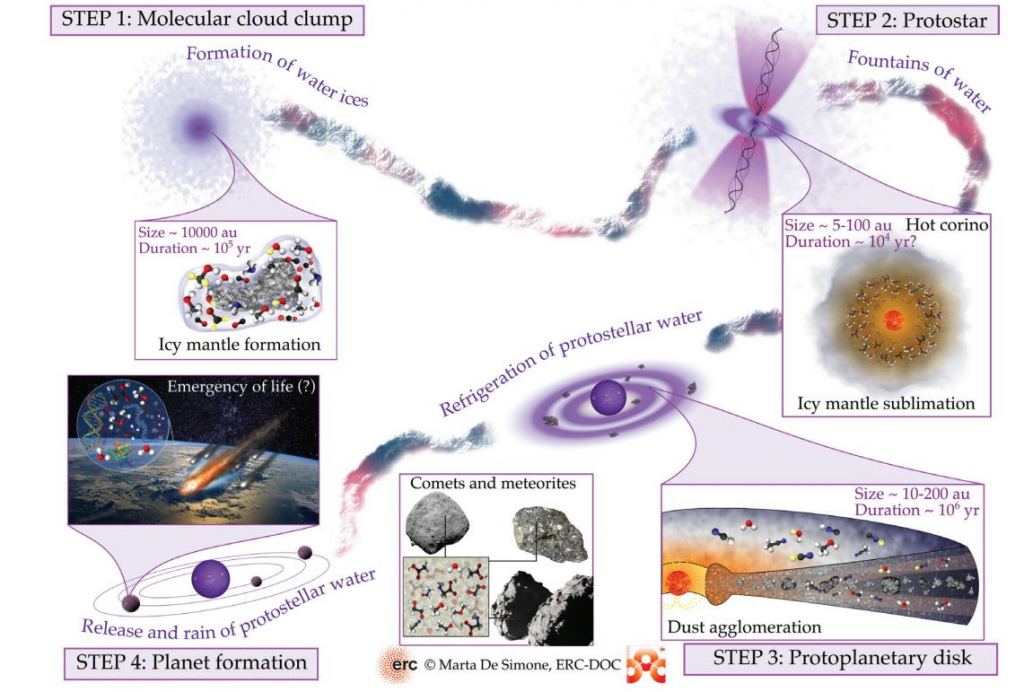
When water ice forms a mantle on dust grains, the authors call it the cold phase, step one in the process they outline in their article.
Gravity begins to exert itself in the cloud as matter clumps in the center. More mass falls into the center of the molecular cloud and starts forming a protostar. Some of the gravity is converted into heat, and within a few astronomical units (AU) of the cloud's center, the gas and dust in the disk reach 100 Kelvin.
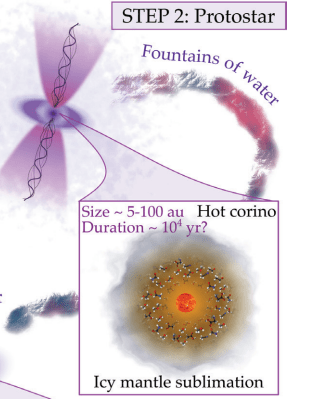
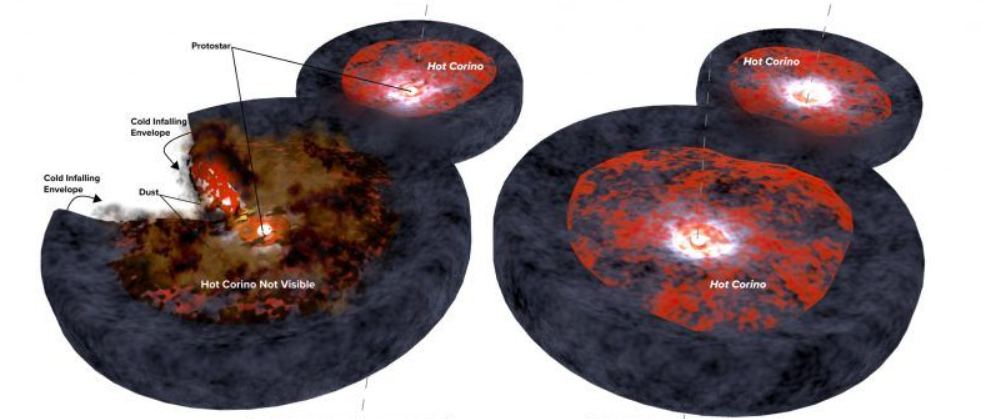
100 K is bitterly cold in Earthly terms, only -173 degrees Celsius. But in chemical terms, it's enough to trigger sublimation, and the ice changes phase into water vapour. The sublimation occurs in a hot corino region, a warm envelope surrounding the cloud's center. Though they also contain complex organic molecules, water becomes the most abundant molecule in corinos.
Water is abundant at this point, though it's all vapour. "... a typical hot corino contains about 10,000 times the water in the Earth's oceans," the authors write.
That's step two in the process outlined by the authors, and they call it the protostar phase.
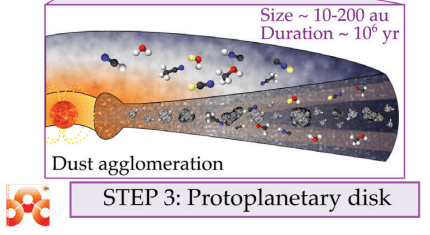
Next, the star begins to rotate, and the surrounding gas and dust form a flattened, rotating disk called a protoplanetary disk. Everything that will eventually become the solar system's planets and other features is inside that disk.
The young protostar is still gathering mass, and its life of fusion on the main sequence is still well in its future. The young star generates some heat from shocks on its surface, but not much. So the disk is cold, and the regions furthest away from the young protostar are the coldest. What happens next is crucial, according to the authors.
The water ice that formed in step one is released into gas in step two but recondenses again in the coldest reaches of the protoplanetary disk. The same population of dust grains is again covered in an icy mantle. But now, the water molecules in that icy mantle contain the history of the water in the Solar System. "Thus, dust grains are the guardians of water inheritance," the authors write.
That's step three in the process.
In step four, the Solar System begins to take shape and resemble a more fully-formed system. All the things we're accustomed to, like planets, asteroids, and comets, start forming and taking up their orbits. And what do they originate from? Those tiny dust grains and their twice-frozen water molecules.
This is the situation we find ourselves in today. While astronomers can't travel back in time, they're getting better at observing other young solar systems and finding clues to the entire process. Earth's water contains a critical hint, too: the ratio of heavy water to regular water.
Some detail is left out of the simple explanation given so far. When water ice forms in step one, the temperature is extremely low. That triggers an unusual phenomenon called super-deuteration. Super-deuteration introduces more deuterium into the water ice than at other temperatures.
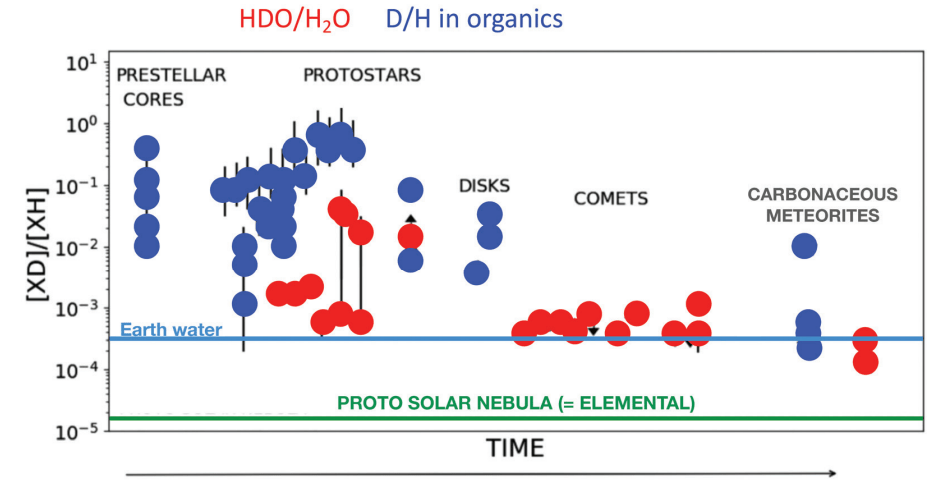
Deuterium was only formed in the seconds following the Big Bang. Not much of it formed: only one deuterium for every 100,000 protium atoms. That means that if the deuterium was evenly mixed with the Solar System's water, the abundance of heavy water would be expressed as 10-5. But there's more complexity to come.
In a hot corino, the abundance changes. "However, in hot corinos, the HDO/H2O ratio is only a bit less than 1/100," the authors explain. (HDO is water molecules containing two deuterium isotopes, and H2O is regular water containing two protium isotopes.)
There's even more extremity. "To make things even more extreme," the authors explain, "the doubly deuterated water D2O is 1/1000 with respect to H2O, namely about 107 times larger than what
would be estimated from the D/H elemental abundance ratio."
The ratios contain such large abundances of deuterium because of super-deuteration. At the moment that ice forms on the surfaces of the dust grains, there's an enhanced number of D atoms compared to H atoms landing on the grain surfaces. The in-depth chemical explanation is beyond the scope of this article, but the conclusion is clear. "There are no other ways to obtain this large amount of heavy water in hot corinos nor in general," the authors write. "Therefore, abundant heavy water is a hallmark of water synthesis in the cold molecular cloud clump during the STEP 1 era."
The important thing so far is that there are two episodes of water synthesis. The first happens when the solar system hasn't formed yet and is only a cold cloud. The second is when planets form. The two happen in different conditions, and those conditions leave their isotopic imprint on the water. Water from the first synthesis is 4.5 billion years old, and the question becomes, "How much of that ancient water reached Earth?"
To find that out, the authors observed the only two things they could: the amount of water overall and the amount of deuterated water. As the authors put it, "... namely, the ratio of heavy over normal water, HDO/H2O."
More than enough water was created to account for Earth's water. Remember that the amount of water in the hot corino was 10,000 times more than Earth's water, and its HDO/H2O ratio is different from the water formed in the initial cloud. How much of the corino water reached Earth? A hint can be found by comparing HDO/H2O values in terrestrial water with those of hot corinos.
Hot corinos are the only place we've observed HDO in any still-forming, solar-type planetary systems. In previous research, scientists compared those ratios with ratios in objects in our Solar System—comets, meteorites, and Saturn's icy moon Enceladus. So they know that Earth's heavy water abundance, the HDO/H2O ratio, is about ten times greater than in the Universe and at the beginning of the Solar System. " 'Heavy over normal' water on Earth is about ten times larger than the elemental D/H ratio in the Universe and consequently at the birth of the Solar System, in what is called the solar nebula," the authors explain.
The results of all this work show that between 1% and 50% of Earth's water came from the initial phase of the Solar System's birth. That's a wide range, but it's still a significant piece of knowledge.
The authors wrap things up in their conclusion. "The water in comets and asteroids (from which the vast
majority of meteorites originate) was also inherited since the beginning in large quantities. Earth likely inherited its original water predominantly from planetesimals, which are supposed to be the precursors of the asteroids and planets that formed the Earth, rather than from the comets that rained on it."
Delivery by comets is another hypothesis for Earth's water. In that hypothesis, frozen water from beyond the frost line reaches Earth when comets are disturbed and sent from the frozen Oort Cloud into the inner Solar System. The idea makes sense.
But this study shows that may not be true.
It still leaves unanswered questions, though. It doesn't explain how all the water reached Earth. But the study shows that the amount of heavy water on Earth is at least the beginning of figuring this out.
"In conclusion, the amount of heavy water on Earth is our Ariadne thread, which can help us to come out from the labyrinth of all possible routes that the Solar System may have taken," they explain.
Earth's water is 4.5 billion years old, just like the article's title says. At least some of it is. According to the authors, planetesimals probably delivered it to Earth, but exactly how that happens isn't clear. There's a lot more complexity that scientists need to sort through before they can figure that out. "The issue is quite involved because the origin and evolution of Earth's water is inevitably connected with other important participants on this planet, e.g., carbon, molecular oxygen, and the magnetic field," the authors write.
These things are all wrapped up together in how life originated and how worlds formed. Water likely played a role in forming the planetesimals that delivered it to Earth. Water likely played a role in sequestering other chemicals, including the building blocks of life, onto rocky bodies that delivered them to Earth.
Water lies at the center of it all, and by showing that some of it dates back to the very beginnings of the Solar System, the authors have provided a starting point for figuring the rest of it out.
"Here, we presented a simplified early history of the Earth's water according to the most recent observations and theories," they write. "A good fraction of terrestrial water likely formed at the very beginning of the Solar System's birth when it was a cold cloud of gas and dust, frozen and conserved during the various steps that led to the formation of planets, asteroids, and comets and was eventually transmitted to the nascent Earth."
"How the final passage happened is another fascinating chapter," they conclude.
More:
- Published Research: We Drink Good 4.5-Billion-Year-Old Water
- Universe Today: Water was Already Here Before the Earth Formed
- Published Research: Origin of Water in the Terrestrial Planets: Insights from Meteorite Data and Planet Formation Models
 Universe Today
Universe Today