The most likely way we will discover life on a distant exoplanet is by discovering a biosignature.
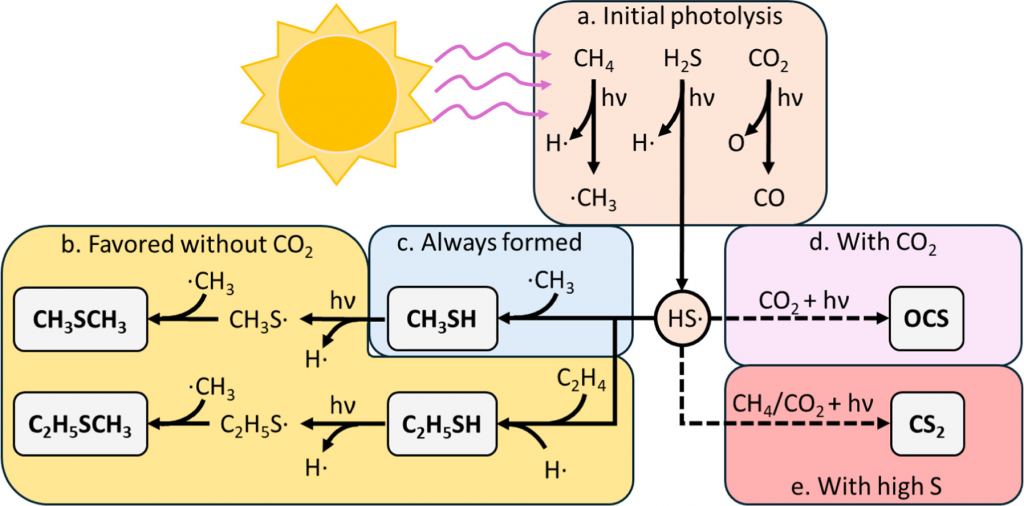
Ideally astronomers would love to find evidence of a really complex molecule such as chlorophyll. But there isn't likely to be tons of chlorophyll in an atmosphere, so the spectral pattern would be faint, and even if it were clear the pattern is complex and hard to distinguish. So astronomers generally focus on simpler but unique molecules. One of these molecules is dimethyl sulfide, (CH3)2S or DMS for short. It is only produced by phytoplankton on Earth, so it would be a strong indicator of life. Or so we thought.
In this new work the team was able to synthesize DMS and other sulfur-based molecules in the lab abiotically. While that doesn't prove the same process can happen in the wild, the team went on to show how DMS could be formed on a world with a thick organic haze. We know such planets exist because Saturn's moon Titan is just such a world. If, for example, Titan happened to be closer to the Sun, the ultraviolet radiation would be significant enough to trigger the chemical reactions necessary to create DMS. If Titan were in Earth's orbit, a distant alien race would detect DMS in the atmosphere of a planet in the Sun's habitable zone. It would look like a slam dunk, but Titan would still be toxic to life as we know it.
But Titan might have some presence of exotic life, which is another conclusion to this study. While the authors show that the presence of DMS or similar molecules wouldn't prove life exists on a world, they argue that it would indicate a strong potential for life. Basically, a warm planet with the kind of rich organic haze in its atmosphere would necessarily have the kind of complex organic molecules life needs to evolve. If DMS exists on a world, then the potential for life exists at the very least.
While this study shows we will need to be careful about treating particular molecules as biosignatures, it also supports what exo-biologists have known for some time. The discovery of life on another world isn't likely going to happen as a single great eureka moment. What is more likely is that a handful of planets will have chemical markers that support the possibility of life. Over time as we find more candidate biomarkers in their atmospheres we will be ever more confident that life exists.
Reference: Reed, Nathan W., et al. " Abiotic Production of Dimethyl Sulfide, Carbonyl Sulfide, and Other Organosulfur Gases via Photochemistry: Implications for Biosignatures and Metabolic Potential." *The Astrophysical Journal Letters* 973.2 (2024): L38.
 Universe Today
Universe Today