Venus is often described as a hellscape. The surface temperature breaches the melting point of lead, and though its atmosphere is dominated by carbon dioxide, it contains enough sulfuric acid to satisfy the comparison with Hades.
But conditions throughout Venus' ample atmosphere aren't uniform. There are locations where some of life's building blocks could resist the planet's inhospitable nature.
Among the rocky planets, Venus has by far the largest atmosphere by volume. So, while its surface is inhospitable, its atmosphere has regions that are the most Earth-like of anywhere else in the Solar System. Scientists have wondered if life could survive in parts of the planet's upper atmosphere, and the discovery of the potential biomarker phosphine (though it was later disproved) generated more interest.
One reason Venus keeps coming up in discussions around habitability is that it's accessible, whereas exoplanets aren't. Venus is easily reached, and we currently have one orbiter in place, the Japanese Akatsuki spacecraft. Three other missions to Venus are planned for the mid-2030s: NASA's Veritas and DAVINCI and the ESA's EnVision.
Nobody is convinced we'll find life on Venus. But the planet can teach us a lot about chemistry and biology and their limits.
In new research, a team of scientists tested different building blocks under Venus-like conditions to see if they can withstand the planet's perilous nature. The research is " Simple lipids form stable higher-order structures in concentrated sulfuric acid. " The lead author is Daniel Duzdevich from the Department of Chemistry at the University of Chicago. The paper is in pre-print now and has been submitted to the journal Astrobiology.
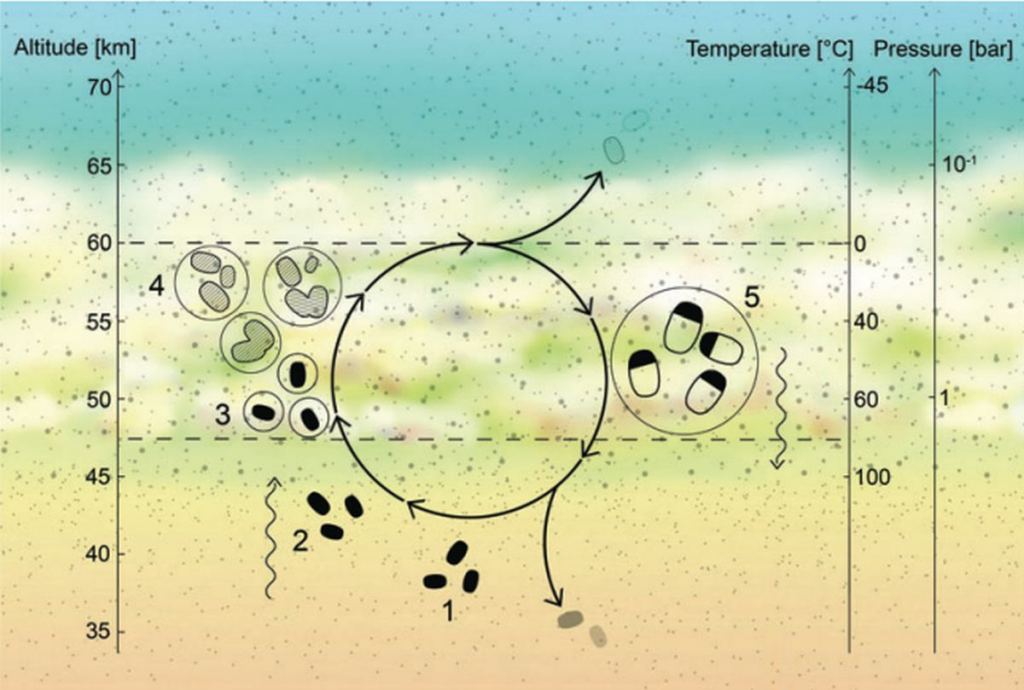
Venus' surface isn't a candidate for habitability. But regions in its atmosphere may be. The issue is that much of Venus' sulfuric acid is concentrated in discrete clouds rather than diffused throughout its atmosphere.
"The Venusian surface is sterilizing, but the cloud deck includes regions with temperatures and pressures conventionally considered compatible with life. However, the Venusian clouds are thought to consist of concentrated sulfuric acid," the authors explain.
They wanted to test if any of life's "fundamental features" could withstand Venus' challenging environment. Can any of life's chemistry resist sulfuric acid?
"Organic chemistry in concentrated sulfuric acid is rarely studied yet surprisingly rich, with recent work supporting the notion that complex organic molecules, including amino acids and nucleobases can be stable in this unusual solvent," the authors write.
If simple organic molecules can remain stable in sulfuric acid, it's an interesting observation in favour of life. But it takes more complexity than that, and that's what this research focuses on.
"One fundamental feature of life is cellularity: the differentiation of "inside" (the contents of a cell, including information, molecules, and all their interactions) and "outside" (the environment), in addition to a mechanism for communication and exchange between the two," Duzdevich and his co-researchers write.
The researchers focused on lipids, the membranes that define cells. Lipids are the foundation of cellular structure, not only as membranes between cells but also as membranes that create distinct parts of the interior of cells. "The cell membrane is especially important in extreme environments because it must help maintain the homeostasis of the intracellular environment against otherwise harsh external conditions," the authors write.
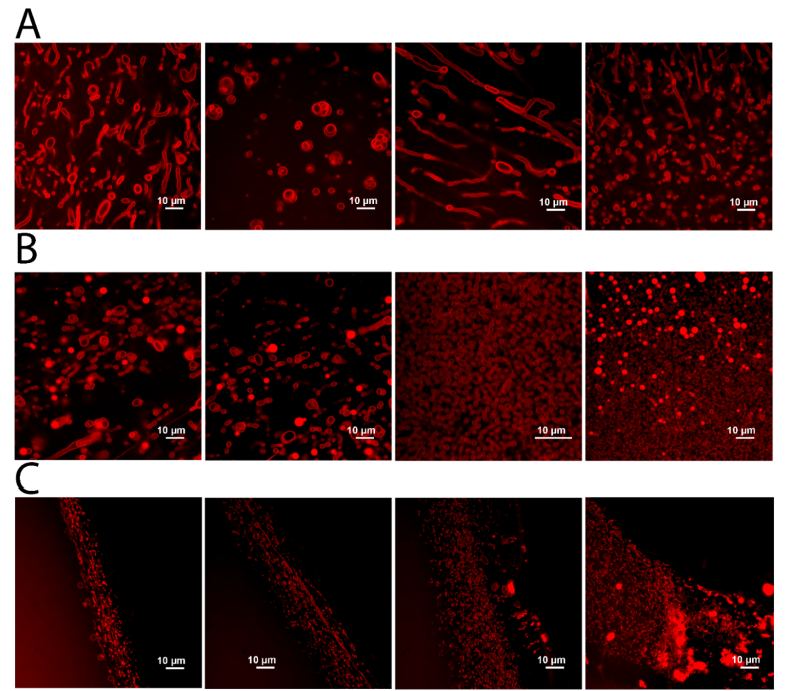
The researchers performed lab experiments to determine whether lipids can withstand Venus' harsh environment. They asked two questions: Can simple lipids resist decomposition by sulfuric acid, and can the lipids form stable higher-order structures like they do in cells?
The researchers placed masses of lipids in vials and exposed them to different concentrations of sulfuric acid and measured each vial at specific intervals. Their results show that some lipids can survive exposure to the acid and even form structures.
Interested readers can explore the detailed chemistry for themselves.
In summary, the results suggest that stable membranes can form and persist in the presence of sulfuric acid. Life uses water as a solvent because it's a polar molecule, can form networks of hydrogen bonds, has a high heat capacity, and, of course, is abundant on Earth. But it's not abundant everywhere.
Critically, this study shows that some aspects of the chemistry of life don't require water as a solvent. Instead, they can tolerate and use sulfuric acid as a solvent. "Here, we show the unexpected stability of complex membranous structures in another polar solvent: concentrated sulfuric acid," the authors write.
What does this mean for exoplanet habitability and astrobiology?
"Concentrated sulfuric acid as a planetary solvent could be widespread on exoplanets, either on exo-Venuses or on other rocky planets that are desiccated as a result of the stellar activity of their host star," the researchers explain.
And, of course, sulfuric acid is present in large amounts at Venus.
"Concentrated sulfuric acid is also present in our immediate planetary vicinity as a dominant liquid in the clouds of Venus, further emphasizing its importance for planetary science, planetary habitability, and astrobiology," the authors write.
The question of whether life could somehow survive in Venus' clouds is one that won't go away. We're new at the astrobiology game, and we're simply not in a position to rule things out. It might seem far-fetched, but science is an evidence game, and evidence can be surprising.
This study doesn't present evidence that can answer the question—big questions like this are answered incrementally—but it does present an intriguing result.
"By demonstrating the stability of lipid membranes in this aggressive solvent, we have taken a significant step forward in exploring the potential habitability of the concentrated sulfuric acid cloud environment on Venus," the authors conclude.
 Universe Today
Universe Today