Will we ever understand how life got started on Earth? We've learned much about Earth's long, multi-billion-year history, but a detailed understanding of how the planet's atmospheric chemistry evolved still eludes us. At one time, Earth was atmospherically hostile, and its transition from that state to a planet teeming with life followed a complex path.
What made Earth so special? Research shows that while Earth is completely different from its neighbouring planets now, in the past, it shared many atmospheric characteristics with modern-day Venus and Mars. How did Earth turn out so different?
A better understanding of Earth's atmospheric journey can help us understand some of the distant exoplanets we've detected. In the near future, new telescopes will be revealing more details of exoplanet atmospheres. Many puzzles await, and some of the solutions to understanding them could be found on ancient Earth.
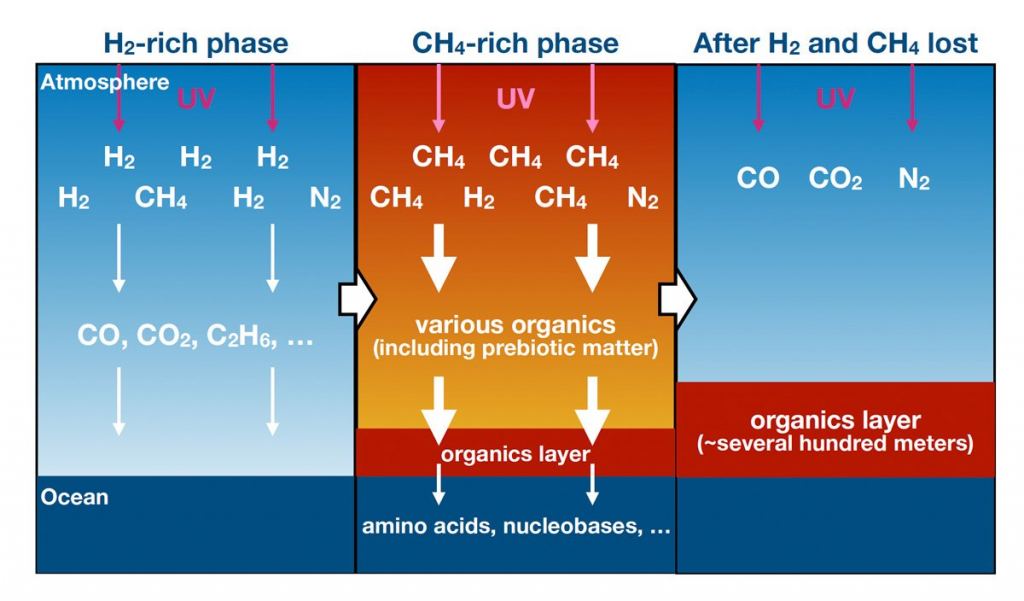
Ancient Earth had a reducing atmosphere, which means that there was a lack of free oxygen. The atmosphere contained reducing gases like hydrogen and methane. These gases quickly react with oxygen and remove it from the atmosphere. Some of those same molecules also react with UV light, and the chemical reactions produce organic molecules.
While that's a general outline of some aspects of ancient Earth's atmosphere, there's a lot of detail that needs to be constrained before a clearer picture emerges of Earth's transformation.
Researchers at Tohoku University, the University of Tokyo, and Hokkaido University have developed a new model of atmospheric chemical reactions that sheds light on how Earth's atmosphere evolved and how the first life may have arisen.
The research is " Self-Shielding Enhanced Organics Synthesis in an Early Reduced Earth’s Atmosphere. " It's published in the journal Astrobiology, and Tatsuya Yoshida from Tohoku University is the lead author.
Before life could appear, Earth needed a good supply of important prebiotic molecules like formaldehyde (H2CO) and poisonous hydrogen cyanide (HCN). These molecules are critical because they can undergo a wide variety of reactions to produce the more complex molecules life requires. They produce amino acids, sugars, and nucleobases, which are the building blocks for DNA and RNA.
Research shows that a highly reduced atmosphere like ancient Earth's is a candidate for producing these important prebiotic molecules, especially if it's above a primordial ocean. Earth's primordial ocean, or proto-ocean, was also much different from the modern ocean. Among other things, it was acidic because of volcanic gases. It was also hot.
"Ancient Earth was nothing like our current home," explains co-author Shungo Koyama, also from Tohoku University. "It was a much more hostile place; rich in metallic iron with an atmosphere containing hydrogen and methane."
The Sun's UV radiation bombarded ancient Earth unimpeded by an ozone layer, driving chemical reactions in the ancient Earth's atmosphere, oceans, and crust.
That much is understood. But what scientists desire is a better understanding of all of the details. "However, the branching ratio between organic matter formation and oxidation remains unknown despite its significance on the abiotic chemical evolution of early Earth," the authors explain.
The researchers developed a photochemical model for a reduced Earth's atmosphere primarily containing H2 and CH4. Their model is based on one that's been successfully applied to Jupiter's atmosphere, the atmospheres of ancient and modern Mars, and runaway greenhouse atmospheres. The model considers 342 separate chemical reactions and also includes atmospheric hydrogen escape and atmospheric mixing.
The young Sun emitted more intense UV radiation than the modern Sun. The UV broke water molecules down into hydrogen and oxygen radicals. Radicals have one unpaired electron, which makes them chemically reactive. Much of the hydrogen escaped to space, while the oxygen did not.
The oxygen radicals combined with methane led to the creation of organic molecules like HCN and H2CO.
Hydrocarbons, such as acetylene (C2H2) and methylacetylene (C3H4), were also present in the atmosphere. These chemicals absorbed some UV, shielding the lower atmosphere from photodissociation. "According to our results, UV absorptions by gaseous hydrocarbons such as C2H2 and C3H4 significantly suppress the H2O photolysis and following CH4 oxidation," the authors explain. The atmospheric methane helped drive the production of organics.
That allowed organic molecules to accumulate into a prebiotic soup, which could've provided the building blocks for life.
"Accordingly, nearly half of initial CH4 possibly becomes converted to heavier organics along with deposition of prebiotically essential molecules such as HCN and H2CO on the surface of a primordial ocean for a geological timescale order of 10-100 Myr," the authors write.
As time went on and the reduced atmosphere evolved, H2CO and HCN were continuously synthesized and accumulated in the ocean. H2CO and HCN are considered to be critical in prebiotic chemistry. According to these results, Earth's early atmosphere was a major source of these important prebiotic molecules. They didn't need to come from meteorites or comets.
The authors calculate that a layer of organic several hundred meters thick may have covered the ocean. "The continuous supply of these prebiotically important molecules could potentially lead to the synthesis of amino acids, nucleobases, sugars, and their polymers," the researchers write.
"There may have been an accumulation of organics that created what was like an enriched soup of important building blocks. That could have been the source from which living things first emerged on Earth," said lead author Yoshida.
The model shows that Earth's early atmosphere was eerily similar to modern-day Mars and Venus. However, Earth evolved into a completely different world. How?
This research doesn't explain it all. But it does deepen our understanding of the evolutionary track Earth followed.
The question becomes, is Earth unique? Or is it a common path that exoplanets in other Solar Systems can follow?
 Universe Today
Universe Today