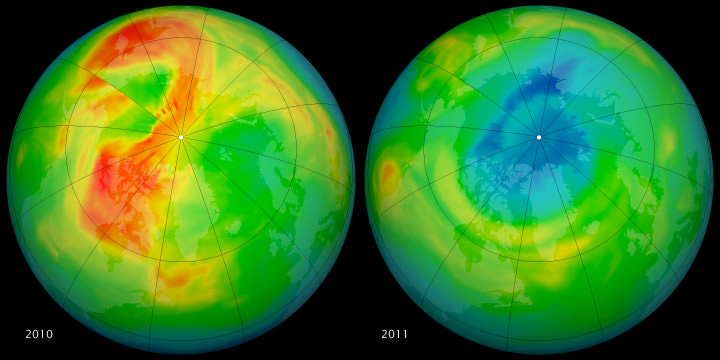
In the past, massive ozone loss over Antarctica has grabbed the headlines. But this year, measurements by several different sources show record levels of stratospheric ozone loss over the Arctic. Scientists say the main reason for the record ozone loss this year is that unusually cold stratospheric temperatures, which have endured later into the season than usual. Scientists say the unusual loss is not catastrophic, but something that needs to be monitored.
The World Meteorological Organization cautioned that people who live in northerly latitudes could get sunburned easier, noting that ozone-depleted air masses extended from the north pole to southern Scandinavia.
The record low temperatures were caused by unusually strong winds, known as the polar vortex, which isolated the atmospheric mass over the North Pole and prevented it from mixing with air in the mid-latitudes.
This has allowed for the formation of polar stratospheric clouds, and the catalytic chemical destruction of ozone molecules occurs on the surface of these clouds which form at 18-25 kilometers height when temperatures drop below -78 C.
[/caption]
This created conditions similar to those that occur every southern hemisphere winter over the Antarctic.
Measurements by ESA’s Envisat satellite, the Ozone Monitoring Instrument (OMI) on NASA’s Aura satellite, and France’s MetOp satellite, as well as observations made since January from the ground and from balloons show all show that 40% of ozone molecules have been destroyed over the Arctic.
Ozone is a protective atmospheric layer found at around 25 km altitude that acts as a sunlight filter shielding life on Earth from harmful ultraviolet rays, which can increase the risk of skin cancer and cataracts in humans and harm marine life.
Stratospheric temperatures in the Arctic usually do vary widely from winter to winter. Last year, temperatures and ozone above the Arctic were very high. The last unusually low stratospheric temperatures over the North Pole were recorded in 1997.
See this link from ESA that shows a animation comparison between 2010 and 2011.
“This depletion is not necessarily a big surprise,” said Paul Newman, an atmospheric scientist and ozone expert at NASA’s Goddard Space Flight Center. “The ozone layer remains vulnerable to large depletions because total stratospheric chlorine levels are still high, in spite of the regulation of ozone-depleting substances by the Montreal Protocol. Chlorine levels are declining slowly because ozone-depleting substances have extremely long lifetimes.”
Ozone “holes” do not form consistently over the North Pole like they do in Antarctica. “Last winter, we had very high lower stratospheric temperatures and ozone levels were very high; this year is just the opposite,” Newman said. “The real question is: Why is this year so dynamically quiet and cold in the stratosphere? That’s a big question with no good answer.”
Scientists will be watching in coming months for possible increases in the intensity of ultraviolet radiation (UV) in the Arctic and mid-latitudes, since ozone is Earth’s natural sunscreen. “We need to wait and see if this will actually happen,” Newman said. “It’s something to look at but it is not catastrophic.”
Scientists are also investigating why the 2011 and 1997 Arctic winters were so cold and whether these random events are statistically linked to global climate change. “In a changing climate, it is expected that on average stratospheric temperatures cool, which means more chemical ozone depletion will occur,” said Mark Weber from the University of Bremen.
Experts say that on a global scale, the ozone layer is still on a long-term course for recovery. But for decades to come, there remains a risk of major ozone losses on yearly or regional scales.
Sources: Nature, ESA, NASA, The Independant Science Daily Earth/Sky Blog

Ozone “holes” do not form consistently over the North Pole like they do in Antarctica. “Last winter, we had very high lower stratospheric temperatures and ozone levels were very high; this year is just the opposite,” Newman said. “The real question is: Why is this year so dynamically quiet and cold in the stratosphere? That’s a big question with no good answer.”
Really no good answer??? http://science.nasa.gov/science-news/science-at-nasa/2011/02mar_spotlesssun/ Nasa has the answer…. SUNSPOTS
For those asking why is this hole forming? What makes this year different?
or why do they keep mentioning the cold air and what does it have to do with the ozone?
The hole exists, because the ozone is being forced out of the area by the cold air.
Ozone O3 is a 3 piece oxygen molecule created by a reaction of sunlight and oxygen in the atmosphere. It is always being created anywhere the sun hits the air, and destroyed by the UV light.
In the antarctic the ice is so thick that a polar vortex always forms during their long night of winter ( a month or so with no sunlight at all.) during that time the cold air pushes all the warm air out and forms almost a wall of cold.
Because the Ozone is formed by sunlight it is warm when created, so is also pushed out. It’s dark during the arctic winter so no Ozone is created there during that time so a hole forms.
Now normally because there is no land mass at the North pole, and very little inside the arctic circle, there is not normally enough ice mass to set up a polar vortex there, but this year it was unusually cold. A vortex formed and then so did an Ozone hole.
In both the arctic and the antarctic the ozone hole collapses once the sun rises again and heats the air enough to break the vortex.
There is a risk though for maybe a few weeks that this cold air mass before it breaks up does not have the ozone levels in it to guard against UV radiation in these very northern cities and towns and they could be at risk to skin cancer from the elevated UV.
Which UV tolerant plants moving northward with global warming will benefit the most?
http://uv.colorado.edu/resources/plants_PDF.pdf
An excerpt from the above link:
“Many algae living in surface waters in lake and the sea are also sensitive to UV. Changes in these algae are likely to effect food supplies for fish, causing a ripple effect all the way up the food chain to humans.”
Summertime upwelling in the Northern Pacific creates the base of a major food chain and that biomass will no doubt be effected by the increased UV. That upwelling and the subsequent explosion of life is one of the ‘fountains of life’ on this planet. We’ll miss that….
Reduce the mechanized fishing fleets of the world by 80%! EDEN NOW!