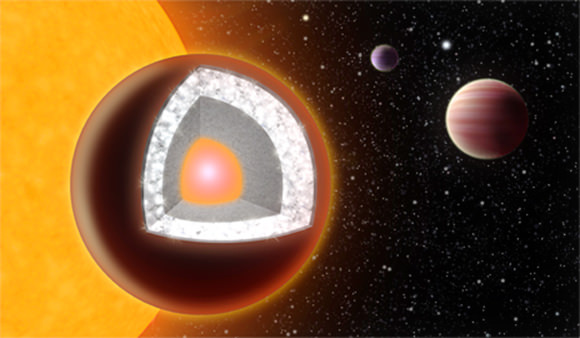
Illustration of 55 Cancri e, a super-Earth that’s thought to have a thick layer of diamond (Yale News/Haven Giguere)
If diamonds are forever then this planet should be around for a very, very long time; it appears to be literally made of the stuff.
 55 Cancri e — an exoplanet discovered in 2004 — is more than twice Earth’s diameter and over eight times more massive, making it a so-called “super Earth.” Earlier this year it made headlines by being the first Earth-sized exoplanet whose light was directly observed via the infrared capabilities of NASA’s Spitzer Space Telescope.
55 Cancri e — an exoplanet discovered in 2004 — is more than twice Earth’s diameter and over eight times more massive, making it a so-called “super Earth.” Earlier this year it made headlines by being the first Earth-sized exoplanet whose light was directly observed via the infrared capabilities of NASA’s Spitzer Space Telescope.
Using information about 55 Cancri e’s size, mass and orbital velocity, as well as the composition of its parent star 55 Cancri (located 40 light years away in the constellation Cancer) a research team led by scientists from Yale University created computer models to determine what the planet is most likely made of.
They determined that 55 Cancri e is composed primarily of carbon (as graphite and diamond), iron, silicon carbide, and possibly some silicates. The researchers estimate that at least a third of the planet’s mass — the equivalent of about three Earth masses — could be diamond.
“This is our first glimpse of a rocky world with a fundamentally different chemistry from Earth. The surface of this planet is likely covered in graphite and diamond rather than water and granite.”
– Nikku Madhusudhan, Yale postdoctoral researcher and lead author
So what would one expect to find on a world made of diamond?
“On this planet there would basically be a thin layer below the surface which will have both graphite and diamond,” Madhusudhan told Universe Today in an email. “But, below that there will be a thick layer (a third of the radius) with mostly diamond. For a large part the diamond will be like the diamond on Earth, except really, really pure.
“But at greater depths the diamond could also be in liquid form,” Madhusudhan added.
Scientists had previously thought that 55 Cancri e might have a lot of water — superheated water, due to the planet’s incredibly high 4,000-degree (F) temperatures — based on the assumption that its composition is similar to Earth’s. But this new research indicates that it doesn’t have much water at all.
“By contrast, Earth’s interior is rich in oxygen, but extremely poor in carbon — less than a part in thousand by mass,” said Kanani Lee, Yale geophysicist and co-author of the paper.
This study shows that we can’t assume that planets in other systems are made of the same stuff that ours is, even if they are of similar size (and also that diamonds aren’t necessarily a valuable commodity on all worlds!)
The team’s paper “A Possible Carbon-rich Interior in Super-Earth 55 Cancri e” was accepted for publication in the journal Astrophysical Journal Letters. Read more on Yale News here.
Top image by Haven Giguere. Inset image shows visible location of 55 Cancri, by Nikku Madhusudhan using Sky Map Online.

Interesting. I wonder if the diamond interior restricts the power of earthquakes on the planet.
Probably not, as seismic waves tend to move more easily through denser material. A subterranean layer of liquid (such as the earth’s outer core) can damp out seismic waves, depending upon thickness and composition. But I would think a thick layer of solid crystalline carbon would actually enhance the transmission of such waves rather than slowing or attenuating them.
I think it might also imply that any tectonics causing such quakes would tend to result in ridiculous amounts of force, owing to the force needed to fracture a pure diamond plate!
Ah, interesting! Thanks for the response.
Here’s the team’s paper (PDF):
A Possible Carbon-rich Interior in Super-Earth 55 Cancri e.
Isn’t this the one that was discovered earlier this year or is this another one?
There was a star that was thought to be composed of diamond as well.
The existence of carbonaceous as opposed to silicious planets have been predicted for some time. It is nice if we have confirmation.
This has obvious effects on habitability. Besides the absence of plate tectonics (IIRC, and shored up by Dave B’s analysis), the geosphere and biosphere would drown in carbon akin to how water worlds drown in water.
In fact, the analogy extends further and it is why I am not specifically worried by this pathway for planets. While there is little evidence for such theories, it has been suggested that there is a “soot line” analogous to the “ice line”. The ice line empties the protoplanetary disks of water close to the star and let the volatile settle where the temperature let it go solid.
Similarly, the soot line would scour the disk of carbon near the star and let hydrocarbons settle as kerogens outside of the ice line. Hence the same models that predict the necessary scarceness of water (for habitability as we know it) close to the stars predict the necessary dominance of silicious minerals.
Carbonaceous terrestrials in the habitable zone would likely be migrants akin to hot Jupiters, deriving from further out in the disk. The hot superEarth 55 Cancri e seems to test such migratory models.
In sum, we are still good for observing many Earth analogs but we have to characterize the planets well to make appropriate statistics for astrobiology purposes. More work for planetary scientists, they got to love this!
The planet has carbon compounds that are analogues of silicon compounds that form the basis for rocks on Earth.
LC
Wait…wouldn’t liquid diamond just be liquid carbon? The existence of diamond depends on a strict crystalline structure of carbon atoms. Mind you, considering I just learned this year that there are 15 types of ice, my knowledge of crystals is clearly limited.
Under very high pressures it is thought that diamond can take a
liquid form.
Under very high pressure, all matter becomes plastic. Sort of both liquid and solid properties at the same.
Amazing information you have threaded up in great presentable way…Thanks a lot..
I am wodering how much gravity is on this world/ could mining opperations land there and mine such materials for ?? purposes and this sort of thing. All way off in our future of course, but then maybe “others” are already doing so. Food for thought.
Very rich planet! It is possible we get those diamonds using robots.