An image of water-filled debris ejected from Cabeus crater about 20 seconds after the 2009 LCROSS impact. Courtesy of Science/AAAS.
Comets? Asteroids? The Earth? The origins of water now known to exist within the Moon’s soil — thanks to recent observations by various lunar satellites and the impact of the LCROSS mission’s Centaur rocket in 2009 — has been an ongoing puzzle for scientists. Now, new research supports that the source of at least some of the Moon’s water is the Sun, with the answer blowing in the solar wind.
Spectroscopy research conducted on Apollo samples by a team from the University of Tennessee, University of Michigan and Caltech has revealed “significant amounts” of hydroxyl within microscopic glass particles found inside lunar soil, the results of micrometeorite impacts.
According to the research team, the hydroxyl “water” within the lunar glass was likely created by interactions with protons and hydrogen ions from the solar wind.
“We found that the ‘water’ component, the hydroxyl, in the lunar regolith is mostly from solar wind implantation of protons, which locally combined with oxygen to form hydroxyls that moved into the interior of glasses by impact melting,” said Youxue Zhang, Professor of Geological Sciences at the University of Michigan.
Hydroxyl is the pairing of a single oxygen atom to a single hydrogen atom (OH). Each molecule of water contains two hydroxyl groups.
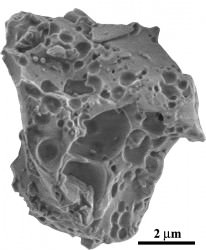 Although such glass particles are widespread on the surface of the Moon — the researchers studied samples returned from Apollo 11, Apollo 16 and Apollo 17 missions — the water in hydroxyl form is not something that could be easily used by future lunar explorers. Still, the findings suggest that solar wind-derived hydroxyl may also exist on the surface of other airless worlds, like Mercury, Vesta or Eros… especially within permanently-shadowed craters and depressions.
Although such glass particles are widespread on the surface of the Moon — the researchers studied samples returned from Apollo 11, Apollo 16 and Apollo 17 missions — the water in hydroxyl form is not something that could be easily used by future lunar explorers. Still, the findings suggest that solar wind-derived hydroxyl may also exist on the surface of other airless worlds, like Mercury, Vesta or Eros… especially within permanently-shadowed craters and depressions.
“These planetary bodies have very different environments, but all have the potential to produce water,” said Yang Liu, University of Tennessee scientist and lead author of the team’s paper.
The discovery of hydroxyl within lunar glasses presents an “unanticipated, abundant reservoir” of water on the Moon, and possibly throughout the entire Solar System.
The study was published online Sunday in the journal Nature Geoscience.
Source: University of Michigan news release.
Inset image: a grain of lunar agglutinate glass from samples returned by Apollo astronauts (Yang Liu)


Yes! Vindicated at last? I have oft posited that Sol makes water… and have in the recent past stated that an ‘overabundance’ of oxygen and hydrogen created during episodes of fusion evolution(s) and elemental upwelling within Sol would then would be transported elsewhere via the solar wind and recombined eventually into water and ice(s)….thereby creating ‘Ice Age’ environments. THIS possibility is looking MUCH more probable now!
Where did the Earth get it’s oceans? Now we know? Got comets? Hmm…. Does the elemental composition of comets reflect Sol’s early evolutionary elemental upheaval(s)?
Did you actually read the article? If you had, you would have seen that it clearly states in the third and fourth paragraphs that:
Which means that protons (hydrogen ions) combine with oxygen atoms in the lunar regolith to form hydroxyl – which is a substructure of the water molecule.
From the above: “According to the research team, the hydroxyl “water” within the lunar glass was likely created by interactions with protons and hydrogen ions from the solar wind.”
My contention is that it is possible that Sol has periodically increased it’s output of molecular oxygen and ionized hydrogen. Oxygen (mass 16) is actually the most abundant heavy element in
the solar wind. At present, there isn’t much of it but as the fusion processes within Sol matured through time, there MAY have been episodes wherein the oxygen output was MUCH higher than presently observed. Abundances of Magnesium and Oxygen is larger
in the slow wind than in the fast wind… so perhaps during episodes of slower solar wind output?
Your contention is misguided and incorrect. Theoretical models show that the CNO cycle is the dominant source of energy in stars more massive than about 1.3 times the mass of the Sun.
So what you are saying is that it is not possible that Sol has gone through multiple episode(s) where the CNO cycle was enhanced toward the end of the sequence because Sol is not massive enough to have induced those fusion processes. Well then, what _might_ have caused those events to occur anyway? Wouldn’t that depend on the original composition of the molecular cloud in which Sol formed? How much ‘metal’ was in that cloud? What catalysts might have been present? Do we know?
The Sun is one of the metal-rich, Population I, group of stars; their high metallicity is the result of previous Population II and Population III stars that had seeded the interstellar medium with heavy elements through the process of nucleosynthesis.
The Ice Ages that you refer to were probably due to the Milankovitch cycles – a combination of orbital shape (eccentricity), axial tilt (obliquity), axial precession, apsidal precession, and orbital inclination.
Ah Sheesh!
Again. Killer mistake (and a debate we’ve had before.)
Again. Hydroxyl is not water!! It is not a “substructure of water” as it behaves chemically so much different from water.
It is OH and is absolutely not the OH- ion. When it gets on the lunar surface, it becomes highly reactive (powerful oxidiser), taking the oxygen from nearby molecules. It then becomes water as H₂O.
Hydroxyl can exist in a vacuum because there are fewer atoms in which it can interact with other molecules.
The article did not claim that hydroxyl is water – the term “water” was placed within quotes to indicate the concept of the term.
Also, we are not talking about the hydroxide ion (OH−) here, we are talking about the neutral hydroxyl radical (•OH).
However, I wasn’t clear in my above statement about “substructure of water”, so I’ve edited it to clarify.
Maybe it is interesting to clarify the difference between *OH and OH- in simple terms. I think that kids and me as none chemists read these posts too and the wikipedia definitions are actually scary to understand.
Most people think of OH as O + H.
I assume that the difference between *OH and OH- is the number of electrons?
Basically, the oxygen atom in a hydroxyl (•OH) radical has 7 electrons in its outer shell (one ‘loaned’ by the hydrogen atom) and a neutral charge, whereas the oxygen atom in the hydroxide (OH−) ion has an extra electron in its outer shell – giving it the ideal 8 – and has a negative charge.
The article title “The Moon’s Water Comes From the Sun”, yet the story is about Hydroxyl, which is not water. Also I have said; “It is OH and is absolutely not the OH- ion.”, So why reflect it back to me?
The error is both the Prof., or his media personnel, and the media (including UT) who are dumbing information so it is comprehensible or relevant. Clearly here they are not a good their job. Frankly you need more people plugging the holes instead of replicating stories from other stories.
For me, and your responses here, it is clear you are not clear on this subject at all. Sorry.
Whatever.
The article describes a form of known reactive sputtering. That is very much my old stomping grounds.
We also know that the problem with terrestrial water (and Moon is in large part Earth material) is to predict the absence, not the presence. Much of Earth and Mars water came with, and this is likely true of Moon as well. So we are discussing different water sources at different locations (bulk of water from the mantle vs surface contributions).
Opposed to these two horses making hoof beats you put one zebra. Or rather a ghost of a zebra, a star doesn’t fusion oxygen until it fusion by the carbon cycle. The relatively young Sun is exclusively fusing hydrogen AFAIK,
On the other hand the Sun contains molecular cloud oxygen (or the system wouldn’t have water), so it has water in the corona:
“Press Release no. 115 — July 17, 1997
University of Waterloo
WATERLOO, Ont. — An international team of scientists, including a University of Waterloo chemistry professor, has conclusively demonstrated that water (actually steam) does exist on the sun, confirming a breakthrough finding made two years ago.
The team used an innovative method to calculate the water spectrum at sunspot temperatures.”
“Or rather a ghost of a zebra, a star doesn’t fusion oxygen until it fusion by the carbon cycle.”
Sorry muted point. This is likely wrong. Fusion is via the CNO-cycle, where all three elements go through a cyclic process. All three elements do not form into three layers inside stars, but are intermixed together. The only Carbon cycle is the collision between three Helium nuclei, and the probability of that is exceedingly small in fusion compared to the CNO-cycle.
As for water on the sun, the reasoning is still not certain, Water is not stable chemically at 6000K, and the chemical dissociation is force to mostly ions. I.e. As;
H₂O ⇔ OH⁻ + O⁺
Water, if formed, would exist and form only beyond the solar corona, which is at millions of degrees.
I do not agree with your statement that Mars water is gone. From scientific data we have until know it looks like there is water everywhere on Mars. Imagine Mars Phoenix Lander which dug in randomly selected location and surprise there is water Ice just few centimeters under ground. If you look on topography of Mars and see wast plains it is very probable that it is simply frozen water. Also if you look on elevated plains you can see how it is melting on sides. You have pictures of gullies with flowing water and steam available. I think all Martian water is still there, just hidden under dust. Mars is just waiting for its chance to thaw. But this time Earth will probably look like Venus….
In 1969 Apollo 12 Lunar Module landed approximately 160 meters from the Surveyor 3 spacecraft, a probe landend on the moon 2 years earlier. The crew retrieved several pieces of the Surveyor and scientists found a small amount of the bacteria Streptococcus mitis in a piece of foam from inside the TV camera. They believed that these bacteria had survived in this location since before launch.
I would not be surprised if some bacterias were still alive today inside the instruments left from the apollo missions on the moon 40 years ago.
By the way, if you want to know more about Apollo 12 and the history of NASA missions I recommend you this app I’ve just found on android market. It’s called NASA Archives and contains more than 20 thousands amazing photos and descriptions about every NASA mission. This is the link https://play.google.com/store/apps/details?id=nasa.archives , just try!
It is known that the bacteria can be simplest predicted by an observed breach of sterile procedure at the precise time to give the observed result.
So we can’t conclude anything from it.
Absolutely spot on.
It’s not really correct to say that one molecule of water contains two hydroxyls…that would be hydrogen peroxide (basically). Water could be described as a protonated hydroxyl. Or maybe the conjugate acid of the OH- ion…
Water doesn’t contain two OH groups any more than ammonia contains multiple amines.