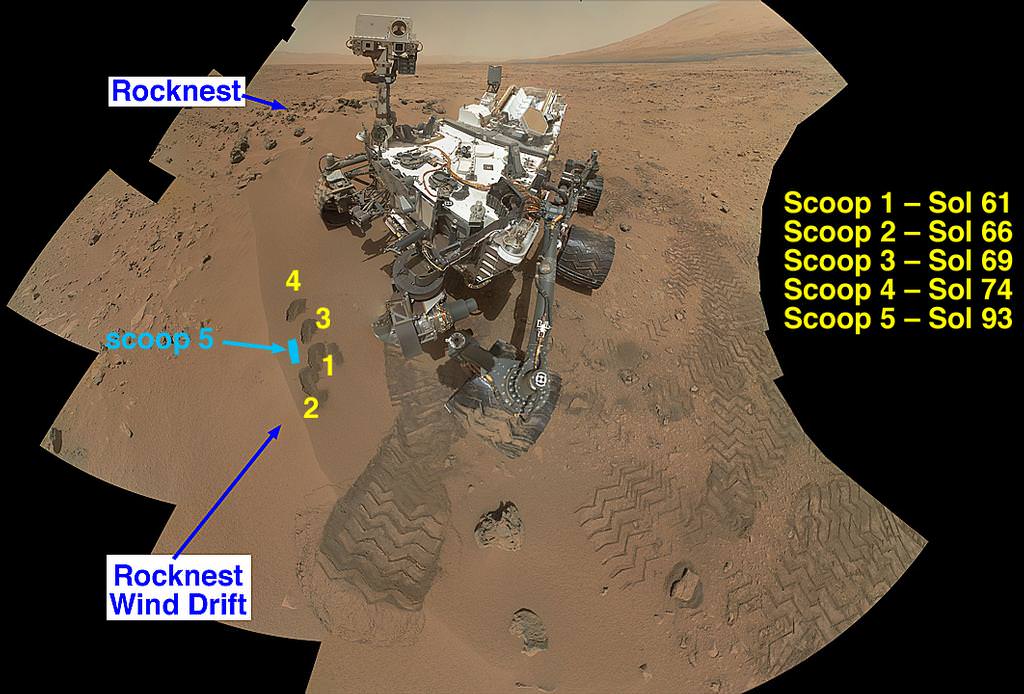
NASA’s Curiosity Mars rover documented itself in the context of its work site, an area called “Rocknest Wind Drift,” on the 84th Martian day, or sol, of its mission (Oct. 31, 2012). The rover worked at this location from Sol 56 (Oct. 2, 2012) to Sol 100 (Nov. 16, 2012). Image credit: NASA/JPL-Caltech/MSSS
The scientists from the Mars Science Laboratory mission had some good news and bad news at the much-anticipated briefing from the American Geophysical Union conference today. The good news is that all instruments are working well on the Curiosity rover, and they have found some potentially interesting compounds … organic compounds. The bad news is they are not sure if the organics are from Mars or not.
“SAM has no definitive detection to report of organic compounds,” said Paul Mahaffy, principal investigator for the Sample Analysis at Mars (SAM) instrument on the Curiosity rover.
This graph compares the elemental composition of typical soils at three landing regions on Mars: Gusev Crater, where NASA’s Mars Exploration Rover Spirit traveled; Meridiani Planum, where Mars Exploration Rover Opportunity still roams, and now Gale Crater, where the Curiosity rover is currently investigating. Credit: NASA/JPL-Caltech/University of Guelph
Interestingly – but not surprisingly – much of the data from the Curiosity rover is similar to previous Mars landers/rovers, such as Viking, the MER rovers and Phoenix. Curiosity’s instruments found chlorine, sulfur and water in Mars soil. Plus, remember the perchlorates that the Phoenix lander found on Mars four years ago? The Sample Analysis at Mars (SAM) instrument on Curiosity has “tentatively” identified perchlorate, which is an oxygen and chlorine compound, which is highly reactive. Reactions with other chemicals heated in SAM formed chlorinated methane compounds, which are one-carbon organics. The MSL scientists said that the chlorine is of Martian origin, but it is possible the carbon may be of Earth origin, carried along from Earth by Curiosity.
Something like this happened previously, where a detection of methane by the SAM suite of instruments turned out to be air that was brought along from Florida, as air leaked into the Tunable Laser Spectrometer (TLS) while the spacecraft was awaiting launch. The initial readings from the TLS, full of methane, were very exciting to the Curiosity scientists until they realized it was from Earth.
And so, with these latest data, the science team wants to make sure these organic compounds truly come from Mars, or if it is from contamination brought along to Mars onboard Curiosity. And one other fly in the ointment is that the organics could also be primordial material from the cosmos delivered to Mars from meteorites, and not be of Martian origin.
But the good news here is that MSL’s suite of instruments should be able to determine the origin of the organics, no matter when they come from.
“This is the first fully integrated measurement on the mission in which every instrument participated in analysis,” said Curiosity Project Scientist John Grotzinger. “And all the instruments working together can tell us if it isn’t originally from Mars… but there’s a complicated decision pathway, and we have to explore each one systematically.”
Grotzinger said they would have to decide whether or not those formation pathways are abiotic or biologic. But that will take a while, as this missions is “moving at the speed of science.”
“This mission is about integrated science,” he said. “No single measurement will produce a hallelujah moment… We are going to pull it all together and take our time and after that if we’ve found something significant we’ll be happy to report that.”
Grotzinger was asked about how his comments a few weeks ago to an NPR reporter were construed as suggesting that an “earth-shaking” discovery had been made by the team, setting off wild speculation of what the rover found.
“What I’ve learned from this is that you have to be careful about what you say,” he said during the briefing, “and even more careful about how you say it. We’re doing science at the speed of science. But we live in a world that’s sort of at the pace of Instagrams. The enthusiasm that we had, that I had, that our whole team has about what’s going on here, I think it was just misunderstood.”
“The great thing about this was, as the days went by, I thought it was terrific this mission has such wide appeal and public interest,” Grotzinger said.
The exciting part, Grotzinger said, is when you have multiple measurements by the instruments that provide similar results. “When we saw SAM replicating results, we knew team would have stuff to chew on for years to come. That’s why we were excited,” he said.
Mahaffy said before the mission, they knew terrestrial contamination could be an issue.
“We’ve gone to great care to address potential confusion that could be caused by terrestrial contamination,” he said. “We have an organic check material along, a silica glass. In the end, we will drill into organic check material that we brought along. If we see same stuff, then it may be terrestrial.”
The Mars Hand Lens Imager (MAHLI) on NASA’s Mars rover Curiosity acquired close-up views of sands in the “Rocknest” wind drift. Credit: NASA/JPL/MSSS
The Curiosity team purposely looked for an area to study that they thought would be rather benign. Curiosity took five scoops of soil from the Rock Nest site, basically a small sand dune. They found sand grains of various sizes, which Ken Edgett from the Hand Lens Imager team described as thick grains “like the salt grains on those big hot pretzels you can get” to much finer material with “grain sizes kind of like artificial sweeteners.”
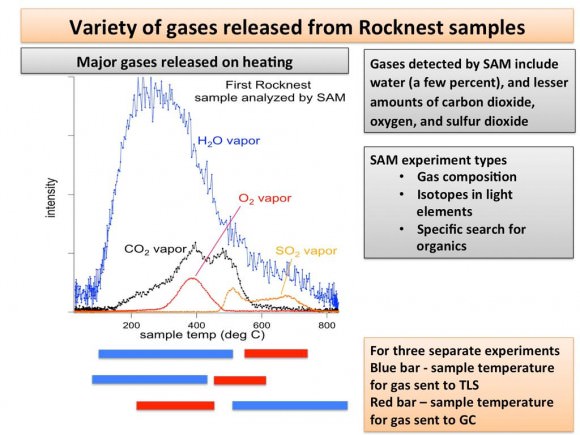
This plot of data from NASA’s Mars rover Curiosity shows the variety of gases that were released from sand grains upon heating in the Sample Analysis at Mars instrument, or SAM. The gases detected were released from fine-grain material, and include water vapor, carbon dioxide, oxygen and sulfur dioxide. Credit: NASA/JPL-Caltech/GSFC
As we reported earlier, the first scoops were used to clean out the chemistry system. The real analysis of a sample came when it was heated to about 500°C and the gases that were released were studied. The most abundant gas was water from water, but the amount of water wouldn’t be enough to support any sort of life, the team said, even though it was higher than expected. And interestingly, the deuterium to hydrogen ratio on the surface of Mars is five times heavier than that in Earth’s oceans.
The scientists said this could be a result of Mars’ gradual loss of atmospheric material, in which lighter isotopes were preferentially lost.
The CheMin instrument found the Rock Nest samples were about half and half common volcanic minerals and non-crystalline minerals such as glass.
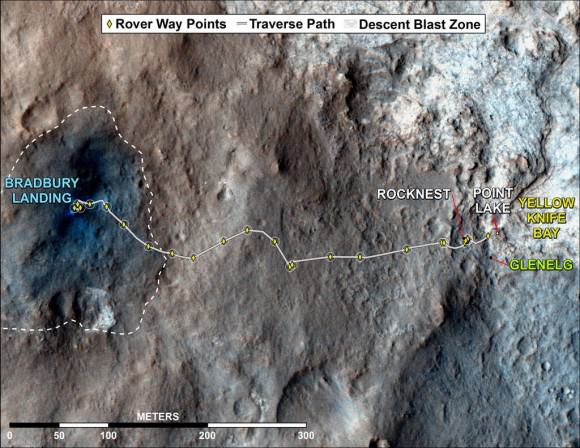
This map shows where the Curiosity has driven since landing at a site subsequently named “Bradbury Landing,” and traveling to an overlook position near beside “Point Lake,” in drives totaling 1,703 feet (519 meters). Credit: NASA/JPL-Caltech/Univ. of Arizona
Beyond that, the team focused on saying this is just the beginning of the mission with lots of time and potential science ahead.
“We’re working on a mission where it’s always going to be difficult to describe in a general way what we’re discovering,” said Grotzinger. “We do want to be very careful about each step along the way. MSL is a mission that is looking for habitable environments. And for that, we would need a source of water, a source of energy and a source of carbon, an essential building block for biological structures.”
Grotzinger said that what tends to happen is that a lot of attention is paid to the third component, and added that the news last week that organics were found on Mercury shows that organics are common in the solar system. And even though the Curiosity rover has already found a water source – the streambed in Gale Crater, along with the water in the soil — the issue is the relative non-abundance of the organics on Mars, so far.
“If we would have found something that was so abundant, that would have been a surprise for us,” he said.



TOP Video News from NASA: http://www.qicknews.de/Forum/viewtopic.php?f=4&t=140&p=4197#p4197
Releases the longest held breath ever… then sighs… and it’s back to biz! This mission IS exciting enough without the distraction the spectacle created? However harmless, it WAS newsworthy and drew lots of attention! One of UT’s early post’s got HUNDREDS of comments! I copied some of the worst and best and sent them off to friends… who like me, thought them hilarious! Cross section of lifeforms found on Earth anyone?
Mixing HOT sulfur with chlorine brews up sulfur mono and dichloride(s) both of which are oxidizing and corrosive, the perchlorates are not much better. Chlorinated methane has been found… I wonder if any other of these will show up?
Ammonium perchlorate, NH4ClO4
Barium perchlorate, Ba(ClO4)2
Caesium perchlorate, CsClO4
Fluorine perchlorate, FClO4
Lithium perchlorate, LiClO4
Magnesium perchlorate, Mg(ClO4)2
Perchloric acid, HClO4
Potassium perchlorate, KClO4
Rubidium perchlorate, RbClO4
Silver perchlorate, AgClO4
Sodium perchlorate, NaClO4
Rocket fuel in there? Or metals extraction as bonus?
Seems they discovered more swamp gas.It’s build-up of hype and subsequent letdown that discourages me from talking about this mission to laypersons and non-scientific minds.
I don’t know what you are expecting from Curiosity, but you shouldn’t pay so much attention to the media hype expecting instant gratification.
Then, you won’t be disappointed.
Since you’ve revealed that you have a scientific mind, you should remember that “good” science plods along at a steady pace, each footstep tries to be placed as sure as possible.
Personally, this mission hasn’t let me down, and I’m still encouraged to talk about it often.
I’m confused… there’s stories all over the rest of the web about the release of news form the JPL update that there ARE organics that were found, just that the actual source needs to be identified…??? What’s up?
For reasons only known to NASA PR they chose to lead with “We have no definitive detection of Martian organics at this point”–note the weasel words ‘definitive’ and ‘Martian.’ Thus confusion was sown, evidenced by the contradictory headlines. As you say, they did find some organics, specifically chloromethanes along with an unspecified 4-carbon chlorinated compound.
Why couldn’t they lead with a straightforward, “we found some simple organics *full stop* but we’ve yet to eliminate the possibility that the carbon is a result of contamination, or possibly brought in by meteorites, or even abiotic or biotic origin on Mars.”
Spend some time looking at the latest Curiosity Mastcam raw images? The recently available high(er) res images show fascinating layered deposits! Some are obviously wind and/or water driven layered volcanic ash deposits? Mons Olympus comes to mind here…
Consistency first:
– The Curiosity SAM wrecks the MERs! On the same scale, one can’t see the error bars.
At the same time, it can be seen that the wind driven soils are truly generic.
– Speaking of consistent soils, they contain impactor and/or volcanic glassy spheres as would be expected.
– It is interesting that the expected perchlorates indeed seems globally distributed, adding to the regionally peroxide oxidants (positive in Viking’s observations IIRC, negative in Phoenix IIRC) that UV light and a short lifetime would conspire to create.
New observations:
– Organics, whether created in the analysis or not, are very scarce. From the count/s diagram of the MS (not shown here), I estimate a couple of species that gives ~ 10^5 counts for ~ 10 s. So ~ 10^7 counts totally.
Given a conservative instrument effectivity of ~ 10^-4 with perchlorates and all, that is ~ 10^11/10^23*100 grams for a ~ 100 au small organic, or ~ 10^-10 g.
For comparison, a typical prokaryote masses ~ 10^-12 g and has ~ 50 % water. So Curiosity observed the organics worth of ~ 200 cells.
– The organics are not so likely derived from heating carbonates. At least calcium carbonate would release its carbon at higher temperature, but they claim carbonates like iron carbonate can match the observations.
– Redox energy resources for life and/or other interesting chemistry! Grotzinger et al notes that the soil has reducing chemical species (sees H2S) as well as the ubiquitous oxidants. This bumps up the energy value of perchlorates.