[/caption]
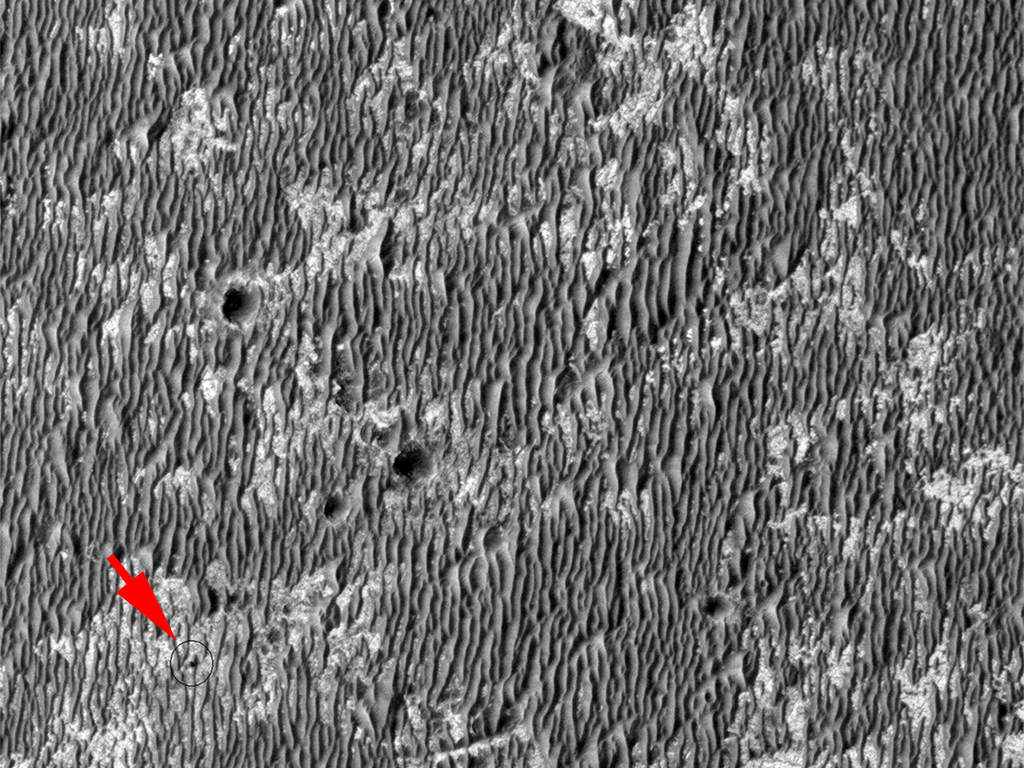
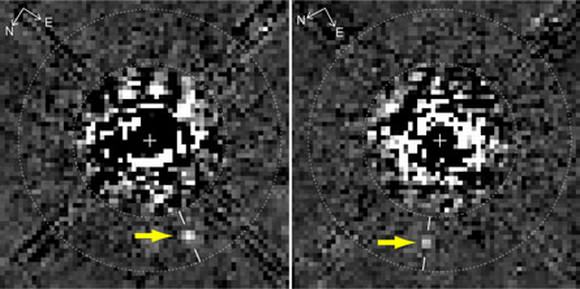
Last month, it was announced that in the few days after the landing of the Phoenix lander in May 2008, the camera attached to the robotic arm captured visual evidence of (what appeared to be) droplets of water, almost like condensation forming on the leg of the lander. In three images dated on sol 8, sol 31 and sol 44 of the mission, the droplets appear to move, in a fluid-like manner. Although a recent publication indicates this oddity could be a water-perchlorate mix (where the toxic salt acts as a potent anti-freeze, preventing the water from freezing and subliming), other members of the Phoenix team are very dubious, saying that there is another, more likely explanation…
One of the key components necessary for the survival of life on Earth is water, especially when the water is in a liquid state. This is an easy proposition on our planet, as the atmospheric pressures and temperatures are just right for the majority of water on Earth to be in a usable liquid state. Should liquid water be discovered on another planet however, where the conditions are often too hot or too cold (or when atmospheric pressure is too low) for water to be found in a liquid state, you’d expect there to be some excitement. When that other planet is Mars, the focal point of the search for basic extraterrestrial life, this excitement will be tempered with intense scrutiny.
In February’s article, Nilton Renno from the University of Michigan and Phoenix mission team scientist, announced results from his team’s research into some odd-looking blobs on one of the lander’s legs. Renno’s hypothesis, to be presented on March 23rd at the Lunar and Planetary Science Conference in Houston (TX), focuses on the possibility that the newly discovered toxic compound, perchlorate, may hold the key to the possibility of liquid water on the Martian surface. We know on Earth, briny (salty) water has a lower freezing point than pure water, and Renno suspects that this might be the case for water on the surface of Mars. However, rather than regular salt, the toxic perchlorate salt is mixed with water in the regolith, allowing it to sustain its liquid state.
Although a very interesting proposition, Renno’s results are based on only photographic evidence of what appears to be blobs of water. Other Phoenix scientists are emphasising that the theory is controversial, citing far simpler answers for the observations.
“There’s a matter of belief at some level,” said Peter Smith from the University of Arizona in Tucson and principal Phoenix investigator. “I can’t say I agree with every statement in the [Renno] paper.”
Michael Hecht, the lead scientist for the instrument that discovered perchlorate in the first place, goes as far to say a perchlorate brine on the Martian surface is very unlikely. Simpler explanations for the apparent dynamic movement of the “liquid” blobs could be attributed to changing shadows. Although perchlorate acts as an efficient “sponge”, condensing water vapour from the surrounding air, the temperatures stated in the paper are actually too warm to form liquid droplets of perchlorate brine.
“I just don’t think it’s the likely explanation,” Hecht said. “It’s just plain old frost, nothing more.”
Looking at the Phoenix images (top), I am a little suspicious about the lifetime of these proposed “liquid” droplets. From sol 8 to sol 44, there is little dramatic change in the locations or sizes of these features. 36 sols of long-term droplets of liquid water seems like a very long time considering the very low atmospheric pressures we are dealing with. Surely liquid brine droplets will dissipate (through evaporation, rather than sublimation) far quicker than 36 sols? Granted, there may be further condensation from the atmosphere (topping up the presence of the liquid), but wouldn’t there be more motion in the blobs if this were the case? This said, I am not familiar with perchlorate brine, so this might well be a characteristic of this cold liquid.
It looks like Renno’s research will make for a very interesting presentation on March 23rd at the Lunar and Planetary Science Conference, sure to provoke a lively debate…
Source: Space.com