[/caption]
NASA scientists studying the comet samples returned by the Stardust spacecraft have discovered glycine, a fundamental building block of life. Stardust captured the samples from comet Wild 2 in 2004 and returned them to Earth in 2006. “Glycine is an amino acid used by living organisms to make proteins, and this is the first time an amino acid has been found in a comet,” said Dr. Jamie Elsila of NASA’s Goddard Space Flight Center. “Our discovery supports the theory that some of life’s ingredients formed in space and were delivered to Earth long ago by meteorite and comet impacts.”
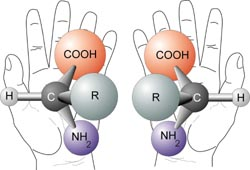
Proteins are a major component of all living cells, and amino acids are the building blocks of protein. Just as the 26 letters of the alphabet are arranged in limitless combinations to make words, life uses 20 different amino acids in a huge variety of arrangements to build millions of different proteins.
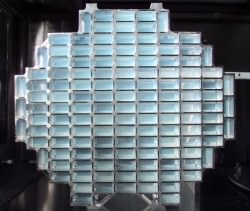
As Stardust passed through dense gas and dust surrounding the icy nucleus of Wild 2 (pronounced “Vilt-2”), special collection grids filled with aerogel – a novel sponge-like material that’s more than 99 percent empty space – gently captured samples of the comet’s gas and dust. The grid was stowed in a capsule which detached from the spacecraft and parachuted to Earth on January 15, 2006. Since then, scientists around the world have been busy analyzing the samples to learn the secrets of comet formation and our solar system’s history.
Earlier, preliminary analysis in the Goddard labs detected glycine in both aluminum foil that lined the collection grids, as well as in a sample of the aerogel. However, since glycine is used by terrestrial life, at first the team was unable to rule out contamination from sources on Earth. “It was possible that the glycine we found originated from handling or manufacture of the Stardust spacecraft itself. We spent two years testing and developing our equipment to make it accurate and sensitive enough to analyze such incredibly tiny samples,” said Elsila. The new research used isotopic analysis of the foil to rule out that possibility.
Isotopes are versions of an element with different weights or masses; for example, the most common carbon atom, Carbon 12, has six protons and six neutrons in its center (nucleus). However, the Carbon 13 isotope is heavier because it has an extra neutron in its nucleus. A glycine molecule from space will tend to have more of the heavier Carbon 13 atoms in it than glycine that’s from Earth. That is what the team found. “We discovered that the Stardust-returned glycine has an extraterrestrial carbon isotope signature, indicating that it originated on the comet,” said Elsila.
Another team member Dr. Daniel Glavin said, “Based on the foil and aerogel results it is highly probable that the entire comet-exposed side of the Stardust sample collection grid is coated with glycine that formed in space.”
The team’s research will be published in the journal Meteoritics and Planetary Science.
Source: NASA