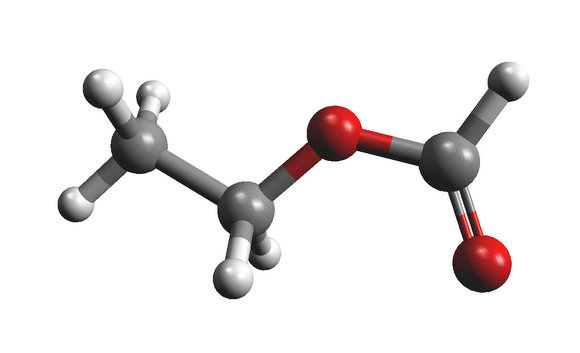
Is your mouth watering? It should be. That molecule at left is called ethyl formate (C2H5OCHO), and it’s partly responsible for the flavors in brandy, butter, raspberries and rum.
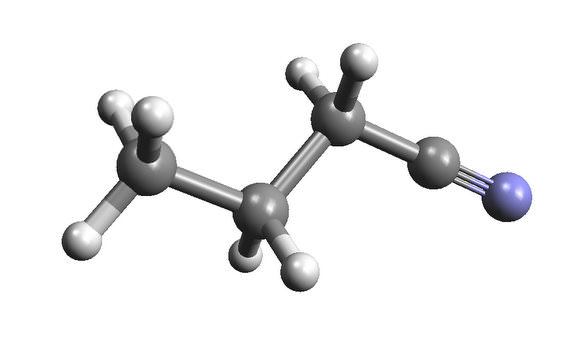
As for this one, it’s a solvent called n-Propyl cyanide (C3H7CN); not so tasty.
They’re both highly complex organics, and they’ve both been detected in space, according to new research — adding mouth-watering evidence to the search for extra-terrestrial life.
The research team hails from Cornell University in Ithaca, New York and the University of Cologne and the Max Planck Institute for Radio Astronomy (MPIfR), both in Germany. Their discoveries represent two of the most complex molecules yet discovered in interstellar space.

To make the observations, the team used the Institut de RadioAstronomie Millimétrique (IRAM) 30m Telescope at Pico Veleta in southern Spain.
Their computational models of interstellar chemistry also indicate that yet larger organic molecules may be present — including the so-far elusive amino acids, believed to be essential for life. The simplest amino acid, glycine (NH2CH2COOH), has been looked for in the past, but has not been successfully detected. However, the size and complexity of this molecule is matched by the two new molecules discovered by the team.
The results are being presented this week at the European Week of Astronomy and Space Science at the University of Hertfordshire, in the UK.
The IRAM was focused on the star-forming region Sagittarius B2, close to the centre of our galaxy. The two new molecules were detected in a hot, dense cloud of gas known as the “Large Molecule Heimat,” which contains a luminous newly-formed star. Large, organic molecules of many different sorts have been detected in this cloud in the past, including alcohols, aldehydes, and acids. The new molecules ethyl formate n-propyl cyanide represent two different classes of molecule — esters and alkyl cyanides — and they are the most complex of their kind yet detected in interstellar space.
Atoms and molecules emit radiation at very specific frequencies, which appear as characteristic “lines” in the electromagnetic spectrum of an astronomical source. Recognizing the signature of a molecule in that spectrum is akin to identifying a human fingerprint.
“The difficulty in searching for complex molecules is that the best astronomical sources contain so many different molecules that their “fingerprints” overlap, and are difficult to disentangle,” says Arnaud Belloche, scientist at the Max Planck Institute and first author of the research paper.
“Larger molecules are even more difficult to identify because their “fingerprints” are barely visible: their radiation is distributed over many more lines that are much weaker,” added Holger Mueller, researcher at the University of Cologne. Out of 3,700 spectral lines detected with the IRAM telescope, the team identified 36 lines belonging to the two new molecules.
The researchers then used a computational model to understand the chemical processes that allow these and other molecules to form in space. Chemical reactions can take place as the result of collisions between gaseous particles; but there are also small grains of dust suspended in the interstellar gas, and these grains can be used as landing sites for atoms to meet and react, producing molecules. As a result, the grains build up thick layers of ice, composed mainly of
water, but also containing a number of basic organic molecules like methanol, the simplest alcohol.
“But,” says Robin Garrod, an astrochemist at Cornell University, “the really large molecules don’t seem to build up this way, atom by atom.” Rather, the computational models suggest that the more complex molecules form section by section, using pre-formed building blocks that are provided by molecules, such as methanol, that are already present on the dust grains. The computational models show that these sections, or “functional groups,” can add together efficiently, building up a molecular “chain” in a series of short steps. The two newly-discovered molecules seem to be produced in this way.
Adds Garrod, “There is no apparent limit to the size of molecules that can be formed by this process — so there’s good reason to expect even more complex organic molecules to be there, if we can detect them.”
The team believes this will happen in the near future, particularly with future instruments like the Atacama Large Millimeter Array (ALMA) in Chile.
Sources: Royal Astronomical Society. The original paper is in press in the journal Astronomy & Astrophysics.
European Week of Astronomy and Space Science
Max Planck Institute for Radio Astronomy
Cologne Database for Molecular Spectroscopy
Reference list of all 150 molecules presently known in space
Cornell University
Institut fuer Radioastronomie im Millimeterbereich (IRAM)
Atacama Large Millimeter Array (ALMA)