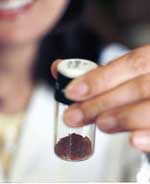
Image credit: UA
While the Cassini spacecraft has been flying toward Saturn, chemists on Earth have been making plastic pollution like that raining through the atmosphere of Saturn’s moon, Titan.
Scientists suspect that organic solids have been falling from Titan’s sky for billions of years and might be compounds that set the stage for the next chemical step toward life. They collaborate in University of Arizona laboratory experiments that will help Cassini scientists interpret Titan data and plan a future mission that would deploy an organic chemistry lab to Titan’s surface.
Chemists in Mark A. Smith’s laboratory at the University of Arizona create compounds like those condensing from Titan’s sky by bombarding an analog of Titan’s atmosphere with electrons. This produces “tholins” ? organic polymers (plastics) found in Titan’s upper nitrogen-methane atmosphere. Titan’s tholins are created by ultraviolet sunlight and electrons streaming out from Saturn’s magnetic field.
Tholins must dissolve to produce amino acids that are the basic building blocks of life. But chemists know that tholins won’t dissolve in Titan’s ethane/methane lakes or oceans.
However, they readily dissolve in water or ammonia. And experiments done 20 years ago show that dissolving tholins in liquid water produces amino acids. So given liquid water, there may be amino acids brewing in Titan’s version of primordial soup.
Oxygen is the other essential for life on Earth. But there is almost no oxygen in Titan?s atmosphere.
Last year, however, Caitlin Griffith, of UA?s Lunar and Planetary Laboratory, discovered water ice on Titan?s surface. (See Titan Reveals a Surface Dominated by Icy Bedrock.) UA planetary scientist Jonathan Lunine and others theorize that when volcanoes erupt on Titan, some of this ice could melt and flow across the landscape. Similar flows could result when comets and asteroids slam into Titan.
Better still, Titan?s water may not immediately freeze because it’s probably laced with enough ammonia (antifreeze) to remain liquid for about 1,000 years, Smith and Lunine noted in a research paper published in last November’s issue of “Astrobiology.”
So although Titan is extremely cold — about 94 degrees kelvin (minus 180 degrees Celsius or minus 300 degrees Fahrenheit) — water may briefly flow across the surface, supplying oxygen and a medium for chemistry, they conclude.
To further understand how all this might work together, Smith’s group is generating tholins in the lab, analyzing their spectroscopic properties, and trying to understand their chemistry.
?We?re trying to learn how the compounds will react with molten water on Titan?s surface, what compounds they?ll make, and, therefore, what we should really be looking for,” Smith explained. “We?re not just looking for atmospheric plastic sitting on the surface, but the result of time and energy input over billions of years.
“We want to know what sorts of molecules have evolved, and whether they’ve evolved along pathways that might provide insights into how biological molecules developed on primordial Earth,? he said.
Mark A. Smith, professor and head of UA’s chemistry department
?Some of what we?ve learned so far in our experiments is that these materials are gross mixtures of incredibly complex molecules,? Smith added. ?Carl Sagan spent the last 10 years of his life studying these compounds in experiments like ours. What we?ve found complements his work. We see the same spectroscopic signatures.”
But Smith’s group also has found that there is a component of these molecules that is very reactive and could easily, within a reasonable time frame, react on the surface of Titan to yield oxygenated compounds.
“And that?s what we?re just starting to unravel now,? Smith said.
?Our work will get much more interesting this fall, in our experiments at the Advanced Light Source of the Lawrence Berkeley Lab,” he added. “We?ll be using a synchrotron to create tholins photochemically, using very energetic photons to break up this Titan gas by vacuum ultraviolet radiation.?
Vacuum ultraviolet radiation hits nitrogen and methane molecules in Titan’s upper atmosphere and blasts them apart. Scientists don’t know if this produces the same kinds of polymers that are formed from an electrical discharge.
?When you can crack nitrogen and methane molecules with light, you might get polymers similar to those formed when an electrical discharge cracks them apart,” Smith said. “Or you may get different polymers. The chemistry is quite complex, and we just don’t know the answers to so many of the simplest questions. But that’s one of the reasons we’ll conduct the experiments at Berkeley.?
The work going on in Smith’s lab is important to scientists on NASA’s Cassini Mission and possible follow-up missions to Saturn. The Cassini orbiter was launched in 1997 and is to launch a probe into Titan’s atmosphere in December. This Huygens probe will float to Titan’s surface next January.
?Titan?s thick orange aerosol haze layer is basically a bunch of organic plastics ? polymers of carbon, hydrogen and nitrogen,” said Smith, head of UA’s chemistry department. “The particulates eventually settle on Titan?s surface, where they produce the organic feedstock for any organic chemistry going on.”
Cassini’s Huygens probe will be the first instrument to actually sample this aerosol. It will give scientists some rudimentary chemical information on this material. But the probe won’t tell them much about organic chemistry at Titan’s surface.
A follow-up mission to Titan that includes a robotic organic chemistry laboratory will give scientists a much more detailed look at the surface. The experiment is being designed by Lunine and Smith in collaboration with researchers from Caltech and NASA’s Jet Propulsion Laboratory.
Lunine leads NASA?s Astrobiology Institute focus group on Titan and is one of three interdisciplinary Cassini mission scientists for the Huygens probe.
?We don?t really know how life formed on the Earth, or on whatever planet it formed,? Lunine said. ?There are no traces left of how it happened on Earth, because all of Earth?s organic molecules have been processed biochemically by now. Titan is our best chance to study organic chemistry in a planetary environment that has remained lifeless over billions of years.?
Original Source: UA News Release