[/caption]
A recent paper published by a NASA scientist claims the discovery evidence of fossil bacteria in a rare subclass of carbonaceous meteorite. The claims are extraordinary, and were the paper published somewhere other than the Journal of Cosmology, (and given an “exclusive preview” on Fox News) more people might be taking this seriously. But, even so, the topic went viral over the weekend.
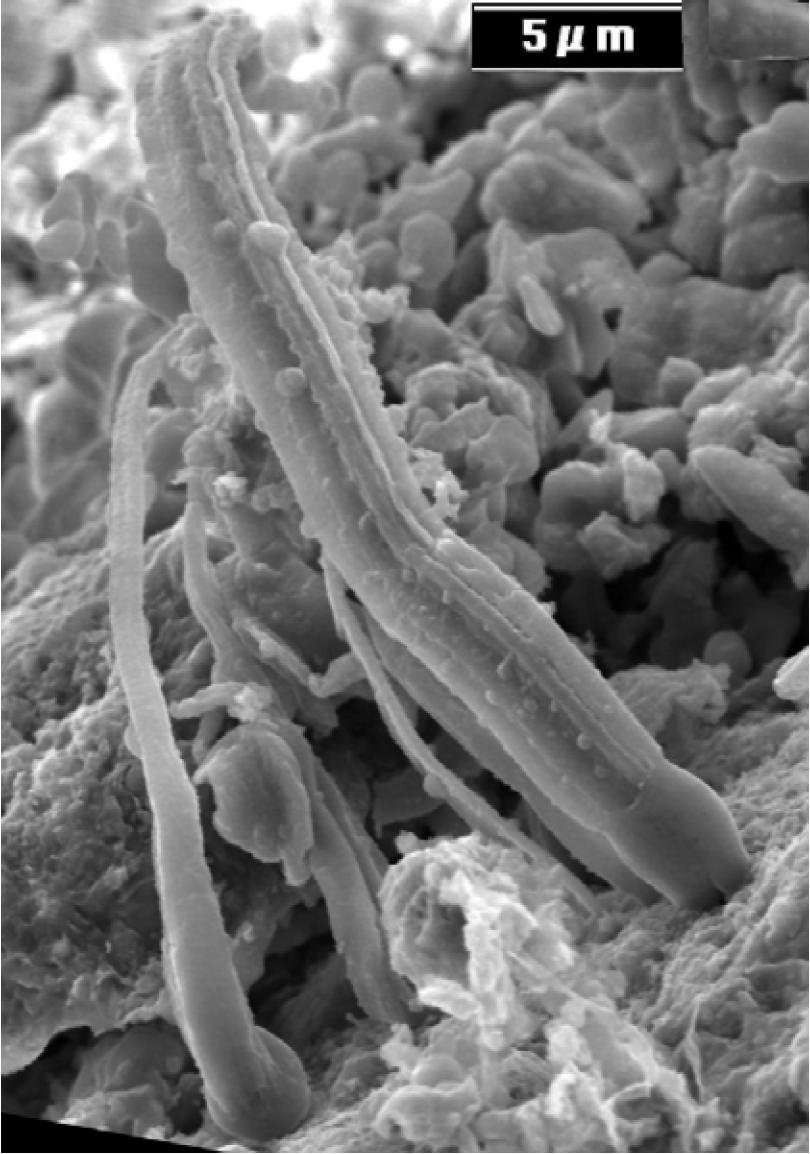
Titled “Fossils of Cyanobacteria in CI1 Carbonaceous Meteorites” and written by NASA scientist Dr. Richard Hoover of the Marshall Space Flight Center, the paper makes the bold claim that meteorites found in France and Tanzania in the 1800s (the Alais, Ivuna, and Orgueil CI1 meteorites) have clear evidence pointing to space-dwelling microbes, with inferences of panspermia — the theory that microbes brought to Earth in comets and meteorites could have started life on our planet. “The implications,” says an online synopsis of the paper, “are that life is everywhere, and that life on Earth may have come from other planets.”
The paper states: “Filaments found in the CI1 meteorites have also been detected that exhibit structures consistent with the specialized cells and structures used by cyanobacteria for reproduction (baeocytes, akinetes and hormogonia), nitrogen fixation (basal, intercalary or apical heterocysts) and attachment or motility (fimbriae).”
Dr. Chris McKay, a planetary scientist and astrobiologist at NASA Ames Research Center, pointed out to Universe Today that Hoover’s claims are “extraordinary, because of the ecological setting implied. Cyanobacteria live in liquid water and are photosynthetic.”
McKay said finding heterocysts (cells formed by some filamentous cyanobacteria) would certainly be indicative of life from an actively thriving environment. “The implication of these results is that the meteorite hosted a liquid water environment in contact with sunlight and high oxygen,” he told Universe Today in an email.
Several scientists from various fields have written commentaries on this, (see astronomer Phil Plait’s take, biologist PZ Myers (from my alma mater) and microbiologist Rosie Redfield (who refuted the “arsenic life” finding late last year), and there’s tons more about this available, and Alan Boyle at MSNBC’c Cosmic Log is keeping a running update) but everyone seems to agree that verifying that the structures — rods and spheres seen in rock — are actually fossilized bacteria is very difficult to do.

There have been previous reports of bacteria in meteorites, but most have turned out to be contamination or misunderstanding of the microscopic structures within rocks (remember the Alan Hills Meteorite claim from 1996 –which is still widely controversial.) It turns out that Dr. Hoover has reported fossil bacteria previously, but none have actually been proven. And, it also turns out that Hoover’s paper was submitted to the Astrobiology Journal in 2007, but the review was never completed.
“Richard Hoover is a careful and accomplished microscopist so there is every reason to believe that the structures he sees are present and are not due to contamination,” McKay said. “If these structures had been reported from sediments from a lake bottom there would be no question that they were classified correctly as biological remains.”
There are two possibilities, McKay said. “One, the structures are not biological but are chance shapes. In a millimeter square area of meteorite there are million possible 1 micron squares. Perhaps any diversity of shapes can be found if searching is extensive.”
Or the second possibility, McKay said is that “the environments on meteorites are, or were, radically different from what we would expect. There are suggestions for how meteorite parent bodies could have sustained interior liquid water. But not in a way that could have the liquid water exposed to sunlight. It also seems unlikely that high oxygen concentrations would be implied.”
There’s also the question of why Hoover would choose to publish in the somewhat dubious Journal of Cosmology, an open access, but supposedly peer-reviewed online journal, which has come under fire for errors found in some of their articles, and for the rather sensational claims made by some of the papers published within.
But word also was released by the Journal of Cosmology that they will cease publication in May 2011. In a press release titled, “Journal of Cosmology To Stop Publishing–Killed by Thieves and Crooks,” (posted by journalist David Dobbs), the press release said that the “JOC threatened the status quo at NASA,” and that “JOC’s success posed a direct threat to traditional subscription based science periodicals, such as “science” magazine; just as online news killed many newspapers. Not surprisingly, JOC was targeted by science magazine and others who engaged in illegal, criminal, anti-competitive acts to prevent JOC from distributing news about its online editions and books.”
UPDATE: NASA has released a statement on Hoover’s paper, saying that “NASA cannot stand behind or support a scientific claim unless it has been peer-reviewed or thoroughly examined by other qualified experts. This paper was submitted in 2007 to the International Journal of Astrobiology. However, the peer review process was not completed for that submission. NASA also was unaware of the recent submission of the paper to the Journal of Cosmology or of the paper’s subsequent publication. Additional questions should be directed to the author of the paper.” – Dr. Paul Hertz, chief scientist of NASA’s Science Mission Directorate in Washington
But Hoover’s work is generating a huge buzz.
The journal’s editor in chief, Rudy Schild of the Harvard-Smithsonian Centre for Astrophysics, said Hoover is a “highly respected scientist and astrobiologist with a prestigious record of accomplishment at NASA. Given the controversial nature of his discovery, we have invited 100 experts and have issued a general invitation to over 5,000 scientists from the scientific community to review the paper and to offer their critical analysis.”
“No other paper in the history of science has undergone such a thorough analysis, and no other scientific journal in the history of science has made such a profoundly important paper available to the scientific community, for comment, before it is published,” Schild added. Those commentaries will be published March 7 through March 10, and can be found here.
Certainly, further review of Hoover’s work needs to be conducted.