[/caption]
The charge of the electron is equivalent to the magnitude of the elementary charge (e) but bearing a negative sign. Since the value of the elementary charge is roughly 1.602 x 10-19 coulombs (C), then the charge of the electron is -1.602 x 10-19 C.
When expressed in atomic units, the elementary charge takes the value of unity; i.e., e = 1. Thus, the electron’s charge can be denoted by -e. Although the proton is much more massive than the electron, it only has a charge of e. Hence, neutral atoms always bear the same number of protons and electrons.
JJ Thomson is the undisputed discoverer of the electron. However, despite all those experiments he performed on it, he could only manage to obtain the electron’s charge to mass ratio. The distinction of being the first to measure the electron’s charge goes to Robert Millikan through his oil-drop experiment in 1909.
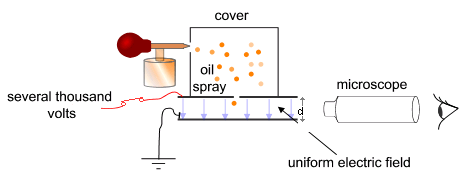
The Millikan Oil-Drop Experiment
Here’s the basic idea. If you know the density and dimensions (thus subsequently the volume) of a substance, it’s going to be easy to calculate its mass and the force that gravity exerts on it, a.k.a. weight. If you recall, weight is just m x g.
Now let’s assume these substances to be charged oil drops. If you subject these drops to gravity alone, they’ll fall freely. However, if they are allowed to fall in a uniform electric field, their trajectory will be altered depending on the direction and magnitude of the field.
If the forces due to the field are directed opposite to gravity, the downward velocity of the particles may decrease. At some point, when the upward force is equal to the downward force, the velocities may even go down to zero and the particles will stay in mid-air.
At this specific instance, if we know the magnitude of the electric field (in N/C, units defining the force per unit charge) and the weight of each particle, we can calculate the force of the electric field on a single particle and finally derive the charge.
Thus, a basic Millikan Oil-Drop Experiment setup will include an enclosure containing falling charged oil drops, a device to measure their radii, an adjustable uniform electric field, and a meter to determine the field’s magnitude.
By repeating the experiment on a large number of oil drops, Millikan and his colleague, Harvey Fletcher, obtained electron charge values within 1% of the currently accepted one.
We have some articles in Universe Today that are related to the charge of the electron. Here are two of them:
Physics World also has some more:
Tired eyes? Let your ears help you learn for a change. Here are some episodes from Astronomy Cast that just might suit your taste:
Sources:
Wikipedia
GSU Hyperphysics
University of Alaska-Fairbanks