Those of you following the Advanced Composite Solar Sail System may have heard that its booms and sail are now deployed. It is receiving light pressure from the Sun to propel it through the Solar System. Like a test pilot in a new aircraft, NASA are now testing out just how it handles. Before deployment, the spacecraft was slowly tumbling and now the controllers will see if they can get it under control and under sail power. The reflectivity of the sail means its an easy spot in the night sky, just fire up the NASA app to find out where to look.
Continue reading “NASA’s Putting its Solar Sail Through its Paces”Photonic Crystals Could Be Exactly What Breakthrough Starshot is Looking For

Light sail technology is a fascinating concept and a step change in rocket propulsion. It may not be big and impressive like the Saturn V, the Space Shuttle or the new Starship rocket but when it comes to travelling among the stars, light sails could just be the answer. And what better material to build the sails from then something that just makes me want to say it over and over again….I talk about photonics crystals. It’s sounds right out of a Star Trek episode but a new paper examines their feasibility.
Continue reading “Photonic Crystals Could Be Exactly What Breakthrough Starshot is Looking For”How Does Light Travel?

Ever since Democritus – a Greek philosopher who lived between the 5th and 4th century’s BCE – argued that all of existence was made up of tiny indivisible atoms, scientists have been speculating as to the true nature of light. Whereas scientists ventured back and forth between the notion that light was a particle or a wave until the modern era, the 20th century led to breakthroughs that showed us that it behaves as both.
These included the discovery of the electron, the development of quantum theory, and Einstein’s Theory of Relativity. However, there remains many unanswered questions about light, many of which arise from its dual nature. For instance, how is it that light can be apparently without mass, but still behave as a particle? And how can it behave like a wave and pass through a vacuum, when all other waves require a medium to propagate?
Theory of Light to the 19th Century:
During the Scientific Revolution, scientists began moving away from Aristotelian scientific theories that had been seen as accepted canon for centuries. This included rejecting Aristotle’s theory of light, which viewed it as being a disturbance in the air (one of his four “elements” that composed matter), and embracing the more mechanistic view that light was composed of indivisible atoms.
In many ways, this theory had been previewed by atomists of Classical Antiquity – such as Democritus and Lucretius – both of whom viewed light as a unit of matter given off by the sun. By the 17th century, several scientists emerged who accepted this view, stating that light was made up of discrete particles (or “corpuscles”). This included Pierre Gassendi, a contemporary of René Descartes, Thomas Hobbes, Robert Boyle, and most famously, Sir Isaac Newton.

Newton’s corpuscular theory was an elaboration of his view of reality as an interaction of material points through forces. This theory would remain the accepted scientific view for more than 100 years, the principles of which were explained in his 1704 treatise “Opticks, or, a Treatise of the Reflections, Refractions, Inflections, and Colours of Light“. According to Newton, the principles of light could be summed as follows:
- Every source of light emits large numbers of tiny particles known as corpuscles in a medium surrounding the source.
- These corpuscles are perfectly elastic, rigid, and weightless.
This represented a challenge to “wave theory”, which had been advocated by 17th century Dutch astronomer Christiaan Huygens. . These theories were first communicated in 1678 to the Paris Academy of Sciences and were published in 1690 in his “Traité de la lumière“ (“Treatise on Light“). In it, he argued a revised version of Descartes views, in which the speed of light is infinite and propagated by means of spherical waves emitted along the wave front.
Double-Slit Experiment:
By the early 19th century, scientists began to break with corpuscular theory. This was due in part to the fact that corpuscular theory failed to adequately explain the diffraction, interference and polarization of light, but was also because of various experiments that seemed to confirm the still-competing view that light behaved as a wave.
The most famous of these was arguably the Double-Slit Experiment, which was originally conducted by English polymath Thomas Young in 1801 (though Sir Isaac Newton is believed to have conducted something similar in his own time). In Young’s version of the experiment, he used a slip of paper with slits cut into it, and then pointed a light source at them to measure how light passed through it.
According to classical (i.e. Newtonian) particle theory, the results of the experiment should have corresponded to the slits, the impacts on the screen appearing in two vertical lines. Instead, the results showed that the coherent beams of light were interfering, creating a pattern of bright and dark bands on the screen. This contradicted classical particle theory, in which particles do not interfere with each other, but merely collide.
The only possible explanation for this pattern of interference was that the light beams were in fact behaving as waves. Thus, this experiment dispelled the notion that light consisted of corpuscles and played a vital part in the acceptance of the wave theory of light. However subsequent research, involving the discovery of the electron and electromagnetic radiation, would lead to scientists considering yet again that light behaved as a particle too, thus giving rise to wave-particle duality theory.
Electromagnetism and Special Relativity:
Prior to the 19th and 20th centuries, the speed of light had already been determined. The first recorded measurements were performed by Danish astronomer Ole Rømer, who demonstrated in 1676 using light measurements from Jupiter’s moon Io to show that light travels at a finite speed (rather than instantaneously).
By the late 19th century, James Clerk Maxwell proposed that light was an electromagnetic wave, and devised several equations (known as Maxwell’s equations) to describe how electric and magnetic fields are generated and altered by each other and by charges and currents. By conducting measurements of different types of radiation (magnetic fields, ultraviolet and infrared radiation), he was able to calculate the speed of light in a vacuum (represented as c).
In 1905, Albert Einstein published “On the Electrodynamics of Moving Bodies”, in which he advanced one of his most famous theories and overturned centuries of accepted notions and orthodoxies. In his paper, he postulated that the speed of light was the same in all inertial reference frames, regardless of the motion of the light source or the position of the observer.
Exploring the consequences of this theory is what led him to propose his theory of Special Relativity, which reconciled Maxwell’s equations for electricity and magnetism with the laws of mechanics, simplified the mathematical calculations, and accorded with the directly observed speed of light and accounted for the observed aberrations. It also demonstrated that the speed of light had relevance outside the context of light and electromagnetism.
For one, it introduced the idea that major changes occur when things move close the speed of light, including the time-space frame of a moving body appearing to slow down and contract in the direction of motion when measured in the frame of the observer. After centuries of increasingly precise measurements, the speed of light was determined to be 299,792,458 m/s in 1975.
Einstein and the Photon:
In 1905, Einstein also helped to resolve a great deal of confusion surrounding the behavior of electromagnetic radiation when he proposed that electrons are emitted from atoms when they absorb energy from light. Known as the photoelectric effect, Einstein based his idea on Planck’s earlier work with “black bodies” – materials that absorb electromagnetic energy instead of reflecting it (i.e. white bodies).
At the time, Einstein’s photoelectric effect was attempt to explain the “black body problem”, in which a black body emits electromagnetic radiation due to the object’s heat. This was a persistent problem in the world of physics, arising from the discovery of the electron, which had only happened eight years previous (thanks to British physicists led by J.J. Thompson and experiments using cathode ray tubes).
At the time, scientists still believed that electromagnetic energy behaved as a wave, and were therefore hoping to be able to explain it in terms of classical physics. Einstein’s explanation represented a break with this, asserting that electromagnetic radiation behaved in ways that were consistent with a particle – a quantized form of light which he named “photons”. For this discovery, Einstein was awarded the Nobel Prize in 1921.
Wave-Particle Duality:
Subsequent theories on the behavior of light would further refine this idea, which included French physicist Louis-Victor de Broglie calculating the wavelength at which light functioned. This was followed by Heisenberg’s “uncertainty principle” (which stated that measuring the position of a photon accurately would disturb measurements of it momentum and vice versa), and Schrödinger’s paradox that claimed that all particles have a “wave function”.
In accordance with quantum mechanical explanation, Schrodinger proposed that all the information about a particle (in this case, a photon) is encoded in its wave function, a complex-valued function roughly analogous to the amplitude of a wave at each point in space. At some location, the measurement of the wave function will randomly “collapse”, or rather “decohere”, to a sharply peaked function. This was illustrated in Schrödinger famous paradox involving a closed box, a cat, and a vial of poison (known as the “Schrödinger Cat” paradox).

According to his theory, wave function also evolves according to a differential equation (aka. the Schrödinger equation). For particles with mass, this equation has solutions; but for particles with no mass, no solution existed. Further experiments involving the Double-Slit Experiment confirmed the dual nature of photons. where measuring devices were incorporated to observe the photons as they passed through the slits.
When this was done, the photons appeared in the form of particles and their impacts on the screen corresponded to the slits – tiny particle-sized spots distributed in straight vertical lines. By placing an observation device in place, the wave function of the photons collapsed and the light behaved as classical particles once more. As predicted by Schrödinger, this could only be resolved by claiming that light has a wave function, and that observing it causes the range of behavioral possibilities to collapse to the point where its behavior becomes predictable.
The development of Quantum Field Theory (QFT) was devised in the following decades to resolve much of the ambiguity around wave-particle duality. And in time, this theory was shown to apply to other particles and fundamental forces of interaction (such as weak and strong nuclear forces). Today, photons are part of the Standard Model of particle physics, where they are classified as boson – a class of subatomic particles that are force carriers and have no mass.
So how does light travel? Basically, traveling at incredible speeds (299 792 458 m/s) and at different wavelengths, depending on its energy. It also behaves as both a wave and a particle, able to propagate through mediums (like air and water) as well as space. It has no mass, but can still be absorbed, reflected, or refracted if it comes in contact with a medium. And in the end, the only thing that can truly divert it, or arrest it, is gravity (i.e. a black hole).
What we have learned about light and electromagnetism has been intrinsic to the revolution which took place in physics in the early 20th century, a revolution that we have been grappling with ever since. Thanks to the efforts of scientists like Maxwell, Planck, Einstein, Heisenberg and Schrodinger, we have learned much, but still have much to learn.
For instance, its interaction with gravity (along with weak and strong nuclear forces) remains a mystery. Unlocking this, and thus discovering a Theory of Everything (ToE) is something astronomers and physicists look forward to. Someday, we just might have it all figured out!
We have written many articles about light here at Universe Today. For example, here’s How Fast is the Speed of Light?, How Far is a Light Year?, What is Einstein’s Theory of Relativity?
If you’d like more info on light, check out these articles from The Physics Hypertextbook and NASA’s Mission Science page.
We’ve also recorded an entire episode of Astronomy Cast all about Interstellar Travel. Listen here, Episode 145: Interstellar Travel.
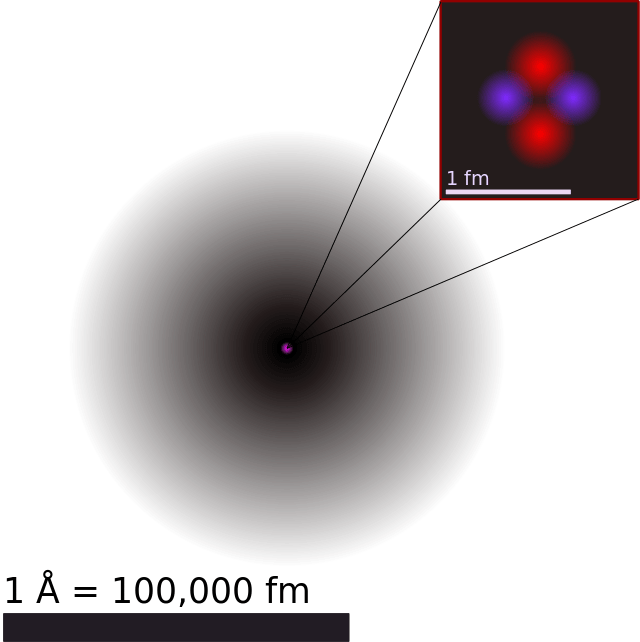
What Are The Parts Of An Atom?
Since the beginning of time, human beings have sought to understand what the universe and everything within it is made up of. And while ancient magi and philosophers conceived of a world composed of four or five elements – earth, air, water, fire (and metal, or consciousness) – by classical antiquity, philosophers began to theorize that all matter was actually made up of tiny, invisible, and indivisible atoms.
Since that time, scientists have engaged in a process of ongoing discovery with the atom, hoping to discover its true nature and makeup. By the 20th century, our understanding became refined to the point that we were able to construct an accurate model of it. And within the past decade, our understanding has advanced even further, to the point that we have come to confirm the existence of almost all of its theorized parts.
Cosmologist Thinks a Strange Signal May Be Evidence of a Parallel Universe
In the beginning, there was chaos.
Hot, dense, and packed with energetic particles, the early Universe was a turbulent, bustling place. It wasn’t until about 300,000 years after the Big Bang that the nascent cosmic soup had cooled enough for atoms to form and light to travel freely. This landmark event, known as recombination, gave rise to the famous cosmic microwave background (CMB), a signature glow that pervades the entire sky.
Now, a new analysis of this glow suggests the presence of a pronounced bruise in the background — evidence that, sometime around recombination, a parallel universe may have bumped into our own.
Although they are often the stuff of science fiction, parallel universes play a large part in our understanding of the cosmos. According to the theory of eternal inflation, bubble universes apart from our own are theorized to be constantly forming, driven by the energy inherent to space itself.
Like soap bubbles, bubble universes that grow too close to one another can and do stick together, if only for a moment. Such temporary mergers could make it possible for one universe to deposit some of its material into the other, leaving a kind of fingerprint at the point of collision.
Ranga-Ram Chary, a cosmologist at the California Institute of Technology, believes that the CMB is the perfect place to look for such a fingerprint.
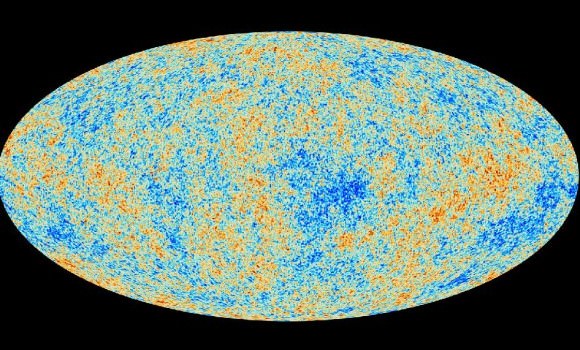
After careful analysis of the spectrum of the CMB, Chary found a signal that was about 4500x brighter than it should have been, based on the number of protons and electrons scientists believe existed in the very early Universe. Indeed, this particular signal — an emission line that arose from the formation of atoms during the era of recombination — is more consistent with a Universe whose ratio of matter particles to photons is about 65x greater than our own.
There is a 30% chance that this mysterious signal is just noise, and not really a signal at all; however, it is also possible that it is real, and exists because a parallel universe dumped some of its matter particles into our own Universe.
After all, if additional protons and electrons had been added to our Universe during recombination, more atoms would have formed. More photons would have been emitted during their formation. And the signature line that arose from all of these emissions would be greatly enhanced.
Chary himself is wisely skeptical.
“Unusual claims like evidence for alternate Universes require a very high burden of proof,” he writes.
Indeed, the signature that Chary has isolated may instead be a consequence of incoming light from distant galaxies, or even from clouds of dust surrounding our own galaxy.
SO is this just another case of BICEP2? Only time and further analysis will tell.
Chary has submitted his paper to the Astrophysical Journal. A preprint of the work is available here.
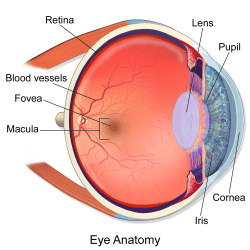
The Journey of Light, From the Stars to Your Eyes
This week, millions of people will turn their eyes to the skies in anticipation of the 2015 Perseid meteor shower. But what happens on less eventful nights, when we find ourselves gazing upward simply to admire the deep, dark, star-spangled sky? Far away from the glow of civilization, we humans can survey thousands of tiny pinpricks of light. But how? Where does that light come from? How does it make its way to us? And how do our brains sort all that incoming energy into such a profoundly breathtaking sight?
Our story begins lightyears away, deep in the heart of a sun-like star, where gravity’s immense inward pressure keeps temperatures high and atoms disassembled. Free protons hurtle around the core, occasionally attaining the blistering energies necessary to overcome their electromagnetic repulsion, collide, and stick together in pairs of two.
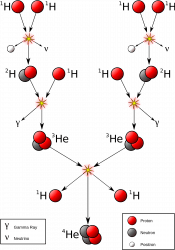
So-called diprotons are unstable and tend to disband as quickly as they arise. And if it weren’t for the subatomic antics of the weak nuclear force, this would be the end of the line: no fusion, no starlight, no us. However, on very rare occasions, a process called beta decay transforms one proton in the pair into a neutron. This new partnership forms what is known as deuterium, or heavy hydrogen, and opens the door to further nuclear fusion reactions.
Indeed, once deuterium enters the mix, particle pileups happen far more frequently. A free proton slams into deuterium, creating helium-3. Additional impacts build upon one another to forge helium-4 and heavier elements like oxygen and carbon.
Such collisions do more than just build up more massive atoms; in fact, every impact listed above releases an enormous amount of energy in the form of gamma rays. These high-energy photons streak outward, providing thermonuclear pressure that counterbalances the star’s gravity. Tens or even hundreds of thousands of years later, battered, bruised, and energetically squelched from fighting their way through a sun-sized blizzard of other particles, they emerge from the star’s surface as visible, ultraviolet, and infrared light.
Ta-da!
But this is only half the story. The light then has to stream across vast reaches of space in order to reach the Earth – a process that, provided the star of origin is in our own galaxy, can take anywhere from 4.2 years to many thousands of years! At least… from your perspective. Since photons are massless, they don’t experience any time at all! And even after eluding what, for any other massive entity in the Universe, would be downright interminable flight times, conditions still must align so that you can see even one twinkle of the light from a faraway star.
That is, it must be dark, and you must be looking up.
The incoming stream of photons then makes its way through your cornea and lens and onto your retina, a highly vascular layer of tissue that lines the back of the eye. There, each tiny packet of light impinges upon one of two types of photoreceptor cell: a rod, or a cone.
Most photons detected under the low-light conditions of stargazing will activate rod cells. These cells are so light-sensitive that, in dark enough conditions, they can be excited by a single photon! Rods cannot detect color, but are far more abundant than cones and are found all across the retina, including around the periphery.
The less numerous, more color-hungry cone cells are densely concentrated at the center of the retina, in a region called the fovea (this explains why dim stars that are visible in your side vision suddenly seem to disappear when you attempt to look at them straight-on). Despite their relative insensitivity, cone cells can be activated by very bright starlight, enabling you to perceive stars like Vega as blue and Betelgeuse as red.
But whether bright light or dim, every photon has the same endpoint once it reaches one of your eyes’ photoreceptors: a molecule of vitamin A, which is bound together with a specialized protein called an opsin. Vitamin A absorbs the light and triggers a signal cascade: ion channels open and charged particles rush across a membrane, generating an electrical impulse that travels up the optic nerve and into the brain. By the time this signal reaches your brain’s visual cortex, various neural pathways are already hard at work translating this complex biochemistry into what you once thought was a simple, intuitive, and poetic understanding of the heavens above…
The stars, they shine.
So the next time you go outside in the darker hours, take a moment to appreciate the great lengths it takes for just a single twinkle of light to travel from a series of nuclear reactions in the bustling center of a distant star, across the vastness of space and time, through your body’s electrochemical pathways, and into your conscious mind.
It gives every last one of those corny love songs new meaning, doesn’t it?
Does Light Experience Time?
Have you ever noticed that time flies when you’re having fun? Well, not for light. In fact, photons don’t experience any time at all. Here’s a mind-bending concept that should shatter your brain into pieces.
As you might know, I co-host Astronomy Cast, and get to pick the brain of the brilliant astrophysicist Dr. Pamela Gay every week about whatever crazy thing I think of in the shower. We were talking about photons one week and she dropped a bombshell on my brain. Photons do not experience time. [SNARK: Are you worried they might get bored?]
Just think about that idea. From the perspective of a photon, there is no such thing as time. It’s emitted, and might exist for hundreds of trillions of years, but for the photon, there’s zero time elapsed between when it’s emitted and when it’s absorbed again. It doesn’t experience distance either. [SNARK: Clearly, it didn’t need to borrow my copy of GQ for the trip.]
Since photons can’t think, we don’t have to worry too much about their existential horror of experiencing neither time nor distance, but it tells us so much about how they’re linked together. Through his Theory of Relativity, Einstein helped us understand how time and distance are connected.
Let’s do a quick review. If we want to travel to some distant point in space, and we travel faster and faster, approaching the speed of light our clocks slow down relative to an observer back on Earth. And yet, we reach our destination more quickly than we would expect. Sure, our mass goes up and there are enormous amounts of energy required, but for this example, we’ll just ignore all that.
If you could travel at a constant acceleration of 1 g, you could cross billions of light years in a single human generation. Of course, your friends back home would have experienced billions of years in your absence, but much like the mass increase and energy required, we won’t worry about them.
The closer you get to light speed, the less time you experience and the shorter a distance you experience. You may recall that these numbers begin to approach zero. According to relativity, mass can never move through the Universe at light speed. Mass will increase to infinity, and the amount of energy required to move it any faster will also be infinite. But for light itself, which is already moving at light speed… You guessed it, the photons reach zero distance and zero time.
Photons can take hundreds of thousands of years to travel from the core of the Sun until they reach the surface and fly off into space. And yet, that final journey, that could take it billions of light years across space, was no different from jumping from atom to atom.
There, now these ideas can haunt your thoughts as they do mine. You’re welcome. What do you think? What’s your favorite mind bending relativity side effect? Tell us in the comments below.
What are Photons
[/caption]
When we think about light we don’t really think about what it is made of. This was actually the subject one of the most important arguments in physics. For the longest time physicists and scientist tried to determine if light was a wave or a particle. There were the physicists of the eighteenth century who strongly believed that light was made of basic units , but certain properties like refraction caused light to be reclassified as a wave. It would take no less than Einstein to resolve the issue. Thanks to him and the work of other renowned physicists we know more about what are photons.
To put it simply photons are the fundamental particle of light. They have a unique property in that they are both a particle and a wave. This is what allows photons unique properties like refraction and diffusion. However light particles are not quite the same as other elementary particles. They have interesting characteristics that are not commonly observed. First, as of right now physicists theorize that photons have no mass. They have some characteristics of particles like angular momentum but their frequency is independent of the influence of mass They also don’t carry a charge.
Photons are basically the most visible portion of the electromagnetic spectrum. This was one of the major breakthroughs Einstein and the father of quantum physics, Planck made about the nature of light. This link is what is behind the photoelectric effect that makes solar power possible.Because light is another form of energy it can be transferred or converted into other types. In the case of the photoelectric effect the energy of light photons is transferred through the photons bumping into the atoms of a giving material. This causes the atom that is hit to lose electrons and thus make electricity.
As mentioned before photons played a key role in the founding of quantum physics. The study of the photons properties opened up a whole new class of fundamental particles called quantum particles. Thanks to photons we know that all quantum particles have both the properties of waves and particles. We also know that energy can be discretely measured on a quantum scale.
Photons also played a big role in Einstein’s theory of relativity. without the photon we would not understand the importance of the speed of light and with it the understanding of the interaction of time and space that it produced. We now know that the speed of light is an absolute that can’t be broken by natural means as it would needs an infinite amount of energy something that is not possible in our universe. So without the photon we would not have the knowledge about our universe that we now possess.
We have written many articles about photons for Universe Today. Here’s an article about how the sun shines, and here’s an article about why stars shine.
If you’d like more info on Photons, check out the Mass of the Photon. And here’s a link to an article about How Gravity Affects Photons.
We’ve also recorded an episode of Astronomy Cast all about the Atom. Listen here, Episode 164: Inside the Atom.
Source:
Wikipedia