[/caption]
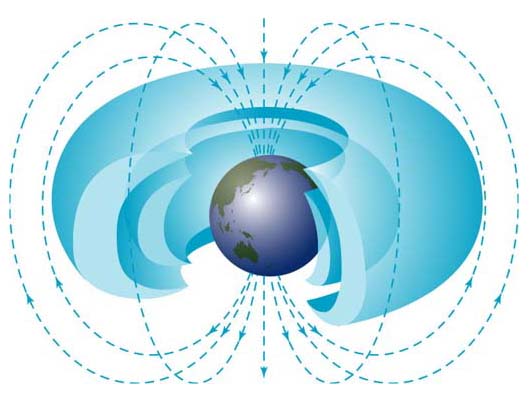
Solar flares, coronal mass ejections, high-energy photons, cosmic rays… space is full of various forms of radiation that a human wouldn’t want to be exposed to for very long. Energized particles traveling into and through the body can cause a host of nasty health problems, from low blood count to radiation sickness to cataracts and cancer… and potentially even death. Luckily Earth’s magnetic field and atmosphere protects us on the surface from much of this radiation, but what about the astronauts aboard the Space Station? How could events such as today’s powerful near-X-class solar flare and last week’s CME affect them, orbiting 240 miles above Earth’s surface?
Surprisingly, they are safer than you might think.

The M8.7-class flare that erupted from the Sun early on Jan. 23 sent a huge wave of high-energy protons Earthward, creating the largest solar storm seen since 2005. The cloud of energetic particles raced outwards through the Sun’s atmosphere at speeds well over a million miles per hour, blowing past our planet later the same day. (More slower-moving charged particles will impact the magnetosphere in the coming days.) We are safe on Earth but astronauts exposed to such radiation could have faced serious health risks. Fortunately, most solar protons cannot pass through the hull of the Space Station and so as long as the astronauts stay inside, they are safe.
Of course, this is not the case with more dangerous cosmic rays.
According to the NASA Science site:
Cosmic rays are super-charged subatomic particles coming mainly from outside our solar system. Sources include exploding stars, black holes and other characters that dwarf the sun in violence. Unlike solar protons, which are relatively easy to stop with materials such as aluminum or plastic, cosmic rays cannot be completely stopped by any known shielding technology.
Even inside their ships, astronauts are exposed to a slow drizzle of cosmic rays coming right through the hull. The particles penetrate flesh, damaging tissue at the microscopic level. One possible side-effect is broken DNA, which can, over the course of time, cause cancer, cataracts and other maladies.
In a nutshell, cosmic rays are bad. Especially in large, long-term doses.
Now the astronauts aboard the ISS are still well within Earth’s protective magnetic field and so are shielded from much of the cosmic radiation that passes through our solar system daily. And, strangely enough, when solar flares occur – such as today’s – the amount of cosmic radiation the ISS encounters actually decreases.
Why?
The solar particles push them away.
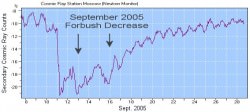
In an effect known as the “Forbush decrease”, magnetically-charged particles ejected from the Sun during flares and CMEs reduce the amount of cosmic radiation the ISS experiences, basically because they “sweep away” other charged particles of more cosmic origin.
Because cosmic rays can easily penetrate the Station’s hull, and solar protons are much less able to, the irony is that astronauts are actually a degree safer during solar storms than they would be otherwise.
And it’s not just in low-Earth orbit, either: Wherever CMEs go, cosmic rays are deflected. Forbush decreases have been observed on Earth and in Earth orbit onboard Mir and the ISS. The Pioneer 10 and 11 and Voyager 1 and 2 spacecraft have experienced them, too, beyond the orbit of Neptune. (via NASA Science.)
Due to this unexpected side effect of solar activity it’s quite possible that future manned missions to the Moon, Mars, an asteroid, etc. would be scheduled during a period of solar maximum, like the one we are in the middle of right now. The added protection from cosmic rays would be a big benefit for long-duration missions since we really don’t know all the effects that cosmic radiation may have on the human body. We simply haven’t been traveling in space long enough. But the less exposure to radiation, the better it is for astronauts.
Maybe solar storms aren’t so bad after all.
Read more about solar radiation and the Forbush decrease on NASA Science here.